Ionic bonds form between _______________ and ___________ elements.
Covalent bonds are bonds between ____________ and ____________ elements.
What are, two non-metals?
The only electrons that are responsible for forming bonds are the electrons located in this shell or cloud?
What are the outer most electrons?
The compounds that go into a chemical reaction are called _________________
What are the reactants?
The term given to the physical property of metals being able to be drawn into wires is called__________
These form when atoms share, give or take electrons with other atoms.
What are bonds?
The chemical formula for dihydrogen monoxide.
What is H2O?
Ionic bonds are bonds formed when atoms ________________ and ____________ electrons.
What is give and take?
If two atoms of the same element for a molecule, such as N2 or O2. These molecules are referred to as ________________ molecules.
What are diatomic molecules?
The dot diagram for H2 would be ?
What is ?
The compounds that are a result of a chemcial reaction are referred to as ____________
What are the products?
Metallic bonds allow metals to be pounded into shapes. This property of metals is referred to as ________
What is Malleability?
This type of bond forms between metals and nonmetals.
What are ionic bonds?
The chemical formula for Sulfur dioxide.
What is SO2?
Why are ionic bonds similar to a magnet?
What are oppositely charged ions feel an attraction to each other.
Covalent bonded compounds tend to have a __________________ boiling and melting point then ionic bonds.
What is lower?
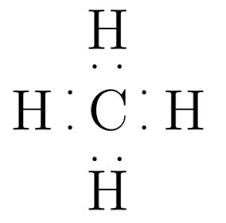
The Lewis dot diagram for CH4 would resemble
The products of a combustion reaction will most likely include these two compounds? You breath them out.
What are carbon dioxide and water?
Metals have the ability to allow electricity and heat to travel easily through them is referred to as ____________.
What is conductivity?
These bonds form between nonmetals and nonmetals?
What are covalent Bonds.
The chemical formula for Aluminum sulfide would be written as ___________
At room temperature, ionic compounds
exist only as this phase of matter. solids.
What are solids?
We defined covalent bonding as electron ____________________.
What is sharing?
This would be the dot diagram of a group one sodium atom.
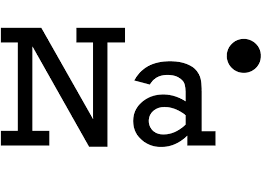
The skeleton equation shown here resembles a __________________ reactions.
A + B → AB
What is an addition reaction?
Metallic bonds are strong and require a great deal of energy to break, and therefore metals have ____________ melting and boiling points.
What is high?
These bonds form when an unequal sharing of electrons occurs, creating oppositely charged poles on the compound.
What are Polar Covalent Bonds?
The number located below the chemical symbol in a chemical formula such as the 2 in H2O is referred to as ______________
What is a subscript?
Ionic bonded compounds tend to have a __________________ boiling and melting point then covalent bonds.
What is Higher?
If the two elements bond and have an unequal sharing of the electrons and a molecule that has two opposite poles, this results in this type of Bond?
What is a polar covalent bond?

This Skelton equation represents this type of reaction.
AB + CD → AD + CB
What is Double replacement reactions?
A metallic substance may be a pure element (e.g. aluminum foil, copper wires), or it may be a mixture of two or more elements. These mixtures are referred to as ___________
What is an alloy?
These bonds occur within metals due to the sea of electrons.
What are Metallic Bonds?
The formula for the compound named, Mercury II (Hg) Iodine would be written as_________________
What is HgI2
Since ionic bonds occur between elements that are located on the opposite side of the periodic table, their bonds tend to be ______________ than covalent bonds.
What are stronger?
Covalent bonds formed between two atoms when both have similar _________________. Or the likely hood to attract and electron.
What is electronegativity?
This dot diagram represents the chemical formula______________
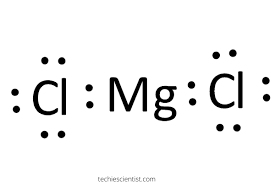
What is MgCl2?
This skeleton reaction represents a ______________ reaction.
AB + C → CB + A
What is single replacement reaction?
Metallic bonds form between atoms that are held together with electrons. This arrangement of negativity charged electrons and positivity charged atoms is referred to as a ___________________
What is a Sea of Electrons?
This member of the British Secret Service, is also referred to as 007.
Who is James Bonds?
The number of Carbon atoms in this chemical formula,
if you had 3 NH4C2H3O2?
What is 6?
At room temperature most covalent compounds exist in phase of matter of a ______________
What is a liquid or a gas?
Having a full outer shell of electrons of 8 is referred to as the __________________ rule.
What is the octet rule?
A synthesis reaction is a reaction in which two or more reactants chemically bond and combine to form a product and heat is released during the reaction. When they release heat, they are considered_______________.
What are exothermic reactions?
The 2's that is located ahead of the element Na and the compound, NaCl in the equation below are referred to as a ________________.
2Na + Cl2 → 2NaCl
What is a coefficient?