Symbol of calcium
What is the Ca?
How many Hydrogen atoms are present in this chemical formula?
3C6H12O6
What is 36?
An element with an unequal number of protons and electrons is called
What is ion?
These negative particles balance the positive protons in an atom.
What are electrons?
Name an element with 3 electron shells.
The answer may vary?
Symbol and name of element with atomic number 19.
What is K, Potassium?
How many atoms are present in this chemical formula? 3Ca(OH)2
What is 15?
An ion with a positive charge
What is a cation?
The number of protons in an atom tells you this.
What is the atomic number?
CO2
What is Carbon Dioxide?
Atomic number of Mg
What is 12?
This is a model of which compound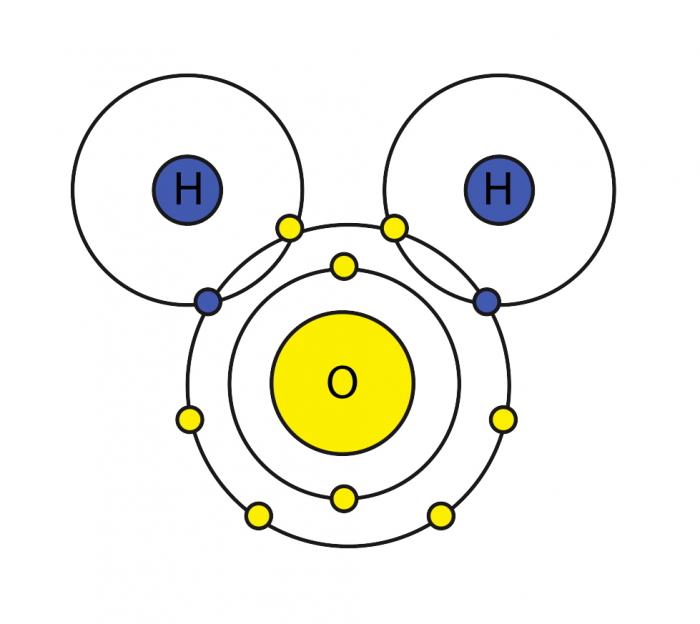
What is water?
Draw the Dot diagram of Carbon.

Sub abtomic parts are present in the nucleus of an atom?
What are protons and neutrons?
What is octet rule?
Atoms will gain, lose, or share electrons to get 8 electrons in their outer shell
What are the elements are # 16, 17, 18
What are Sulfur, Chlorine, and Argon?
Write the chemical formula of ammonia
What is NH3 ?
Explain the octet rule and duplet rule.
Octet rule to complete 8 valence electrons.
Duplet rule to complete 2 valence electrons on the first shell only.
35Cl2+, how many electrons does it have?
What are 15 electrons?
NiCl2 + (NH4)2S -----> NiS + NH4Cl
How many nitrogen atoms are there on the reactant side?
What is 2?
Name 2 elements with 8 valence electrons
What are Neon and Argon?
Which has fewer Oxygen atoms?
2Fe2O3 or
Al2(SO4)3
The first equation.
Draw and explain ionic bonding using NaCl and an example.

15O- . Identify the number of its protons, neutrons, and electrons
What are p-8, n-7, and e-9?
Compare cation and anion.
Cation: An atom that loses electrons and becomes positively charged. Anion: An atom that gains electrons and becomes negatively charged.
