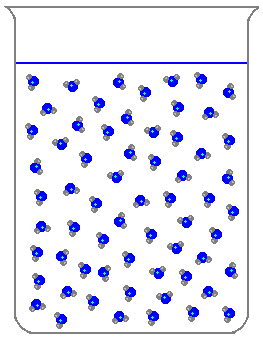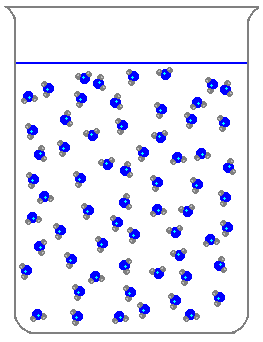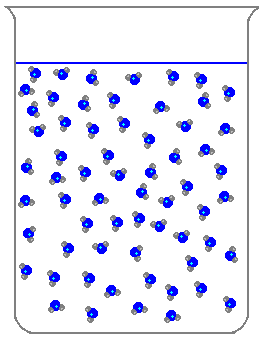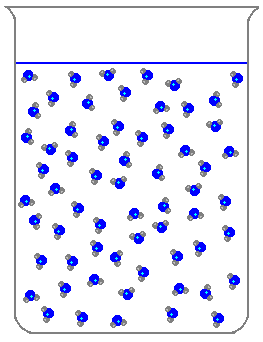
Which of the following is a characteristic of solids?
A. They have a definite shape and volume.
B. They take the shape of their container.
C. They expand to fill their container.
A. They have a definite shape and volume
What is a substance that is not mixed with any other substance?
A. Pure Substance
B. Element
C. Chemical Change
A. Pure Substance
A(n) _______________________ cannot be broken down into any other substance.
A. mixture
B. element
C. compound
B. Element
What does cooler, denser material do?
float or sink
sink
A chemical change:
A. DOES result in a new substance.
B. DOES NOT result in a new substance.
A. Does result in a new substance
How do particles in a solid differ from those in a liquid?
A. Particles in a solid move freely, while those in a liquid are fixed.
B. Particles in a solid are tightly packed and vibrate in place, while those in a liquid can move past each other.
B. Particles in a solid are tightly packed and vibrate in place, while those in a liquid can move past each other.
Coffee is a...
A. Homogeneous
B. Heterogeneous
C. Pure Substance
Which of the following is the most metallic element in period 3?
A. Sodium (Na)
B. Argon (Ar)
C. Silicon (Si)
A. Sodium
Which box has a higher density?
Box A
When two substances are mixed, they might form a new substance. Which of the following would indicate that a new substance has been formed?
A. The volume of the mixture increases.
B. One of the substances dissolves.
C. The temperature of the mixture decreases.
A. The temperature of the mixture decreases.
This diagram represents a _____.
Mixture
True or False:
Heterogeneous: A mixture in which the substances that make up the mixture are not evenly mixed.
True
Isra is analyzing the properties of several samples of elements to find out which sample is a metalloid. Which set of properties most likely belongs only to a metalloid?
A. is a gas and has low electrical conductivity
B. is brittle and forms acidic compounds
C. has a high luster and is very brittle
C. Has a high luster and is very brittle
Put the liquids in order from most dense to least dense?
4, 3, 2, 1
Which example indicated a chemical change has occurred?
A. boiling water bubbles when heated
B. sugar is not visible when dissolved in water
C. paper changing color and releasing gas and smoke when burned
C. paper changing color and releasing gas and smoke when burned
A student is given a sample of an unknown substance. He is asked to determine if it is classified as a metal, a metalloid, or a nonmetal. He discovered that the unknown element conducted some heat and electricity, had a shiny luster, and broke easily. This element is most likely a??
Metalloid
What is this?
Pure Substance or Mixture
Mixture
Why are rare earth elements considered valuable despite not being very rare?
A. They are found in large quantities in the Earth's crust
B. They are easy to extract and purify
C. They are crucial for building a lot of technology and are in high demand
C. They are crucial for building a lot of technology and are in high demand
A can of Coke will sink in a bowl of water. A can of Diet Coke will float in a bowl of water. What does this tell you about the density of the Diet Coke?
Compare its density to bowl of water.
The diet coke is less dense then the bowl of water
Observe the chemical equation shown here. What evidence of a chemical reaction is present when 2NO is added to O2?
Brown Gas
What state of matter does this picture show?
Liquid
A ______________ mixture is one that does not have a uniform composition.
heterogenous
Why are scientists motivated to find new ways to recycle rare earth elements?
A. Because they are not in high demand
B. Because they are in high demand and the demand is expected to increase
C. Because they are not useful in green technologies
B. Because they are in high demand and the demand is expected to increase
Which object has the highest density in this picture?
Picture C
What evidence can you use to infer that a chemical reaction has occurred here?
A dark solid formed on the zinc metal