Write the formula for copper (II) hydroxide
CuO
3018Ar+2
How many neutrons, protons, and electrons does this element have and wha tis its atomic and mass number?
neutrons: 12
protons:18
electrons: 16
atomic #: 18
mass #: 30
Give the first Law of Thermodynamics
In an isolated system, the total amount of energy (including heat energy) is conserved.
(energy can be transferred from one state to another but not destroyed)
It is found that 1.431g of element X contains 7.509x10^21 atoms. Calculate the atomic mass of X.
1.431g X *(6.023 x 10^23 atoms/1 g X) *(1gX/7.509x10^21 atoms)=114.8 g of X
Is helium gas used to fill a balloon a mixture?
No, it is a substance because it's made of the same atoms
Write the name for Mg(OH)2
Magnesium (II) Hydroxide
What is the Lewis structure for water and is it polar or non polar?
polar

What are the three types of systems? Explain all
Open system: matter and energy can exchange freely with surroundings
closed system: matter cannot freely exchange with surroundings but energy can
isolated system: matter cannot freely exchange with surroundings
Sorbose C6H12O6 is used in making Vitamin C. Determine the number of carbon atoms in 25 moles of sorbose.
25 moles C6H12O6 * (6.023 x 1023 molecules/ 1 mol C6H12O6) * (6 atoms C/1 molecule C6H12O6)=
9.0 x 1023 atoms of carbon
Is milk turning sour a chemical or physical change?
It is a chemical change because the properties of the milk are changing
For each of the following compounds, decide whether the compound is expected to be ionic or molecular:
a) Li3N
b) As4O6
c)ClF3
a) ionic compound (made of metals and nonmetals)
b) molecular compound (made of nonmetals)
c) molecular compound (made of nonmetals)
The elements in which period usually exceed the octet rule and why?
Period 3 because their D orbitals are available.
Heat energy equal to 32,000J is applied to a 800g brick whose specific heat is 2.45 J/gC.
What is the change in temperature of the brick?
32,000J=(2.45 J/gC)(800g)(Delta T)
Delta T= 16.3 degrees C
Pure copper may be produced by the creation of copper (I) sulfide with oxygen gas as follows:
Cu2S(s) + O2(g) --> 2Cu(s) + SO2(g)
What mass (in Kg) of copper (I) sulfide is required in order to prepare 0.640 kg of copper metal?
0.640 kg Cu= 640 g Cu
640 g Cu * (1 mol Cu/63.55g/mol Cu)*(1 mol Cu2S/2 mol Cu) * (159.16 g Cu2S/1 mol Cu2S)= 801 Cu2S= 0.801 kg Cu2S.
Difference between a theory and scientific law?
Theory is an idea backed up by evidence but a scientific law is an idea that is backed up by loads of evidence and has been proven and agreed up by multiple scientists
Balance the following formula:
__NH3 (g) + __O2 --> __NO2 (g) + __H2O
(Hint: write out how many moles of each element are on each side)
4 NH3 (g) + 7 O2 --> 4 NO2 (g) + 6 H2O
Draw Lewis Structure for BH2- and describe its molecular geometry
bent

A metal object with a mass of 200g, originally at 78.1 degrees C, is dropped into 100g of water originally at 22.1 degree C. The final temperature of both the pellet and the water is 33.7 degrees C. Calculate the specific heat capacity (in J/gC) of the pellet.
(200g metal)(33.4 degrees C-78.1 degrees C)(X)=(100 g water)(33.7 degrees C-22.1 degrees C)(4.184J/gC)
solve for x
x= 0.547 (J/gC)
SO2 reacts with H2S as follows:
2H2S + SO2 --> 3S + 2H2O
In one experiment 7.50g of H2S reacts with 12.75g of SO2. Determine which of the two reactants is the limiting reactant.
7.50 g H2S x (1 mol H2S/ 34.076g H2S) x (2mol H2O/2 mol H2S) =0.220 mol H2O
12.75 g SO2 x (1 mol SO2/64 g SO2) x (2 mol H2O/1 mol SO2) = 0.398 mol H2O
H2S is limiting reactant
A solution is prepared by dissolving table salt, sodium chloride in water at room temperature. Assuming there is no significant change in the volume of water during the preparation of the solution, how would the density of the solution compare to that of pure water?
The solution with pure water would be less dense because it wouldn't not have the excess salt that the other solution has.
Balance the following oxidation-reduction reactions by the half-reaction method. Label oxidizing agents and reducing agents.
CuCl2(aq) + Al(s) --> AlCl3 (aq) +Cu(s)
3 CuCl2(aq) +2 Al(s) --> 2 AlCl3 (aq) + 3 Cu(s)
ionic: 3 Cu +2 + 6 Cl- + 2 Al0 -->2Al+3 + 6 Cl- + 3Cu0
reducing agent: Al
oxidizing agent: Cu
What is the Lewis structure for ClF4+. State the electron and molecular geometry.
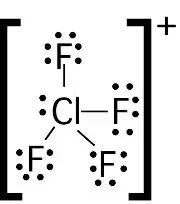
trigonal bipyramidal (electron) and see saw (molecular)
What is the change in enthalpy for the following reaction?
3CH4 (g) + 4O3 (g) --> 3CO2 (g) + 6H2O (g)
Substance=Heat of Formation
CH4=-74.87 (kJ/mol)
O3=+142.7 (kJ/mol)
CO2=-393.5 (kJ/mol)
H2O=-241.8 (kJ/mol)
[(3*-393.5)+(6*-241.8)]-[(2*-74.87)+(4*142.7)]=-2977.5 kJ/mol
A compound containing only carbon, hydrogen, and oxygen is subjected to elemental analysis. Upon complete combustion, a 0.4266-g Sample of the compound produced 0.5518 g of CO2 and 0.2259g H2O. What is the empirical formula of the compound?
CH2O (go over problem on board)
Water has a density of 0.997 g/cm^3 at 25 degrees C; ice has a density of 0.971 g/cm^3 at -10 degrees C. If a soft drink bottle whose volume is 1.50 L is completely filled with water and then frozen to -10 degrees C, what volume does the ice occupy?
d=m/v
1.5L=1500cm^3
0.997 g/cm^3=m/1500cm^3 -->m(water)=1495.5g
0.971g/cm^3=1495.5g/v -->v=1540.165cm^3
1540.165cm^3=1.54L