What are the three subatomic particles?
Proton, Neutron, Electron
*Bonus* What are the charges on each particle?
Tell me what each piece of information means in this picture ![]()
Metal and Nonmetal
Do ionic molecules require prefixes?
NO!
Bonus: Why?
Do covalent molecules require prefixes?
YES!
How does adding or removing an electron affect an atom?
Increase or decrease an atom's negative charge
When bonding, gaining and losing electrons can stabilize an element by giving both atoms a full octet. (ex, Na with 1 electron to lose and Cl with room for 1 electron)
Which colors show the nonmetals? 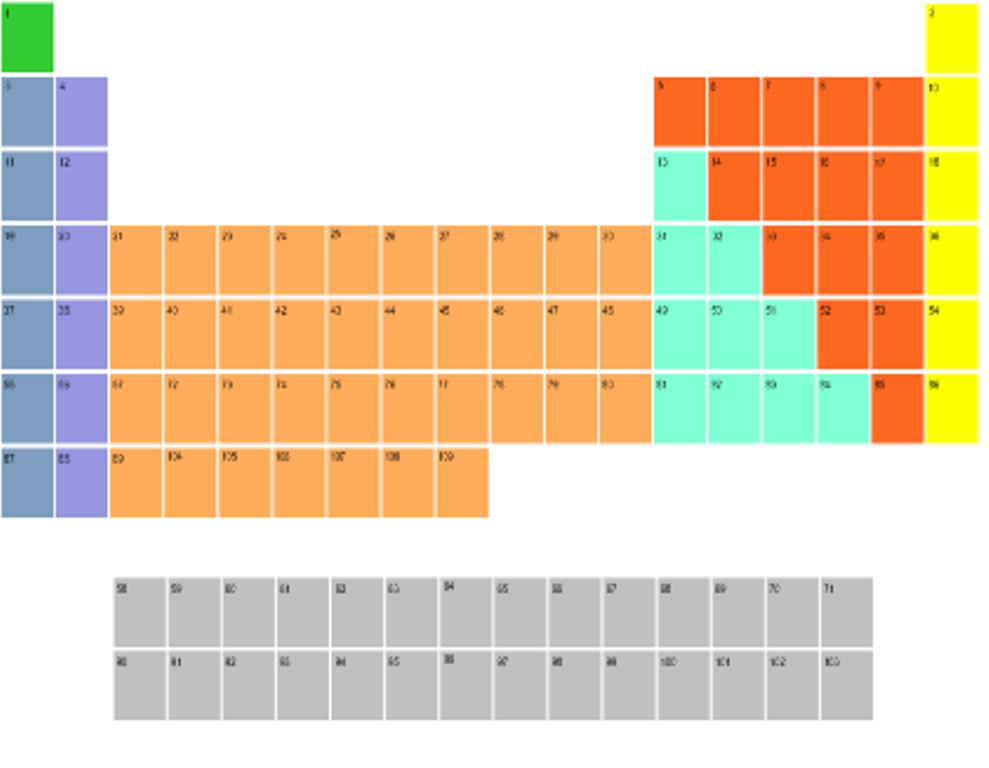
What types of elements form covalent compounds?
non-metal and non-metal
Name: ZnBr2
Zinc Bromide
What Suffix comes at the end of these compound's names?
-ide
What is an ion?
Metal atoms lose electrons and nonmetal atoms gain electrons to form ions with the same number of electrons as the nearest noble gas (ex, Na loses 1 to be like Neon)
Which color are the noble gases?
are the noble gases?
Yellow!
Identify this compound as ionic or covalent
IONIC
FeCl3
When should you use the prefix mono-
Only when there is one of the second element. First element does not require mono- if there is just one. Ex - Carbon dioxide, CO2
Which subatomic particle determines the identity of an element?
PROTONS. Changing #of protons changes the identity of an element
(change in electrons changes charge, change in neutrons creates isotopes)
Where are your Alkali Metals and Halogens? 
Alkali Metals: Groups 1 and 2
Halogens: Group 17, left of noble gases
Identify this compound as ionic or covalent: 
COVALENT
What is the formula for Sodium Carbonate?
Na2CO3
Write the formula for Dihydrogen Monoxide
H2O
What is an isotope?
Which colors have elements of potentially multiple charges?
Orange and Teal
Identify this compound as ionic or covalent
IONIC
What do ROMAN NUMERALS mean when they are in a compound?
CHARGE of the cation/metal/first element
Name: P4O10 compund
Tetraphosphorous decaoxide