Label the variables and their units in the following equation:
E=(hc)/lamda
E= energy of a photon (J)
h= Planck's Constant (J*s)
c= Speed of light (m/s)
lamda= wavelength (m)
These are the four quantum numbers.
What are n, l, ml, and ms?
This is the nuclid symbol.
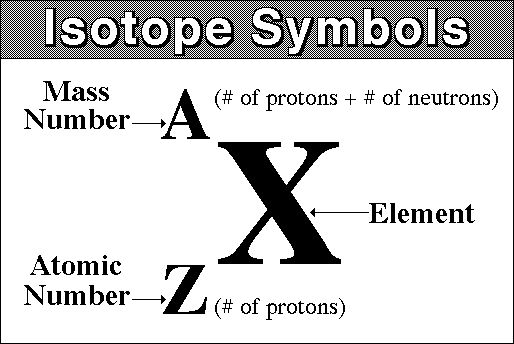
1 atm, 25oC or 298 K
What is STP?
When two wave frequencies converge to create a wave with a higher amplitude than the originals, we call it this.
What is constructive interference?
In Schrodinger's famous thought experiment, we are asked to think about how, when observed, this particle will either have wave or particle behavior.
What is the electron?
What is the first principle quantum number for each orbital shape? (include the f orbital)
p->2
d->3
f->4
These are the assumptions we make about gases when using the kinetic molecular theory. (Doesn't need to be in the form of a question)
1. The size of the particles is small compared to the total system volume
2. The average particle kinetic energy is proportional to the temperature->velocity of particles increase when temp increases
3. Particle collisions with each other and the container walls are completely elastic (no energy is lost in the system, perfect transfer of momentum)
This is what the deltas mean in the uncertainty principle.
What is the uncertainty in the position and speed of a particle? It does not mean the change in position or speed. It means how much we don't know about these characteristics.
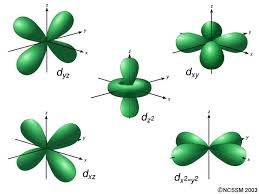
These are the five configurations for the d-orbital subshell. (Draw and label)
DOUBLE JEOPARDY!!!!!
Determine the number of protons, neutrons, and electrons in Bi-209.
What are 83 pro, 126 neu, and 83 elect?
When determining the pressure of gas that we had collected over water, we have to take into consideration this partial pressure.
What is the vapor pressure of water?
Sometimes, when light shines on a material, the material emits electrons.
What is the photoelectric effect?
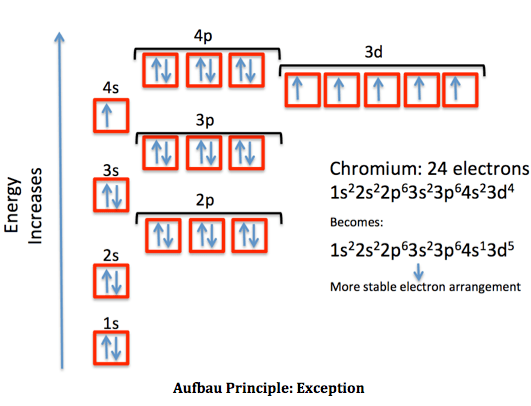
Draw the electron orbital configuration diagram of chromium starting with the 1s orbitals.
Arrange the following in terms of increasing first ionization energy. How would this change if we were to arrange them in terms of increasing atomic radius? (Doesn't need to be in the form of a question)
Sn, P, Cs, F, Sr
Cs<Sr<Sn<P<F
If we rearranged it from greatest to smallest atomic radius, then the order would be reversed.
You increase the pressure on an elastic, closed container by a factor of three and decrease the temperature by a fourth. How does the new volume compare to the original volume? DOUBLE JEOPARDY!!!!
1/4 V1