This geometry includes lone pairs and bonds.
What is the electron geometry?
This is the hybridization of a linear electron geometry.
What is sp?
Draw a labeled molecular orbital diagram. You do not need to include atomic orbitals.
1 atm, 0oC or 273.15 K
What is STP?
This specific geometry has one lone pair and two bonding domains. (Include both mg and eg)
What is trigonal planar bent?
This scientist introduced the concept of hybridization. He also went crazy, thought vitamins would make you live forever, then died of cancer. (Interesting facts XD)
Who is Linus Pauling?
A molecule with all electrons paired and is repelled by a magnetic field.
What is a diamagnetic molecule?
or
What is diamagnetic?
Which of these gases would you expect to behave the most ideally? (Does not need to be in the form of a question)
a)N2
b)NO2
c)Ar
d)H2
H2
Reasoning: Smallest and one of the most inert.
Which of the following molecules are polar?
CH3F, CH4, CF4, CO2, H3COH
a) CH3F, CF4, CO2, H3COH
b) CH3F, H3COH
c) CH3F, CF4
d) CO2, H3COH
e) All of these molecules are polar
What is b?
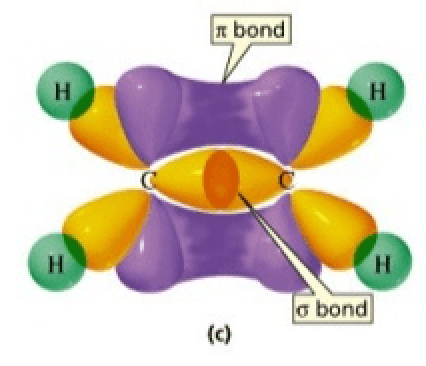
Draw the pi and sigma bonds in for this molecule. (Doesn't need to be in the form of a question) How many sigma and how many pi bonds are there?
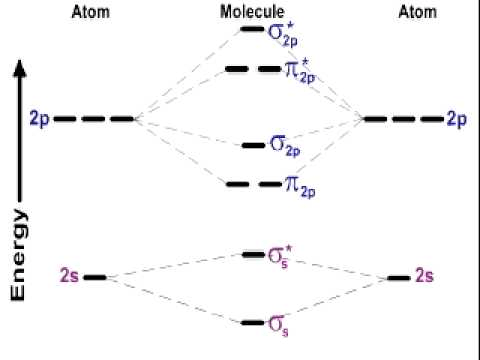
This is the molecular orbital diagram for a particular set of atoms:
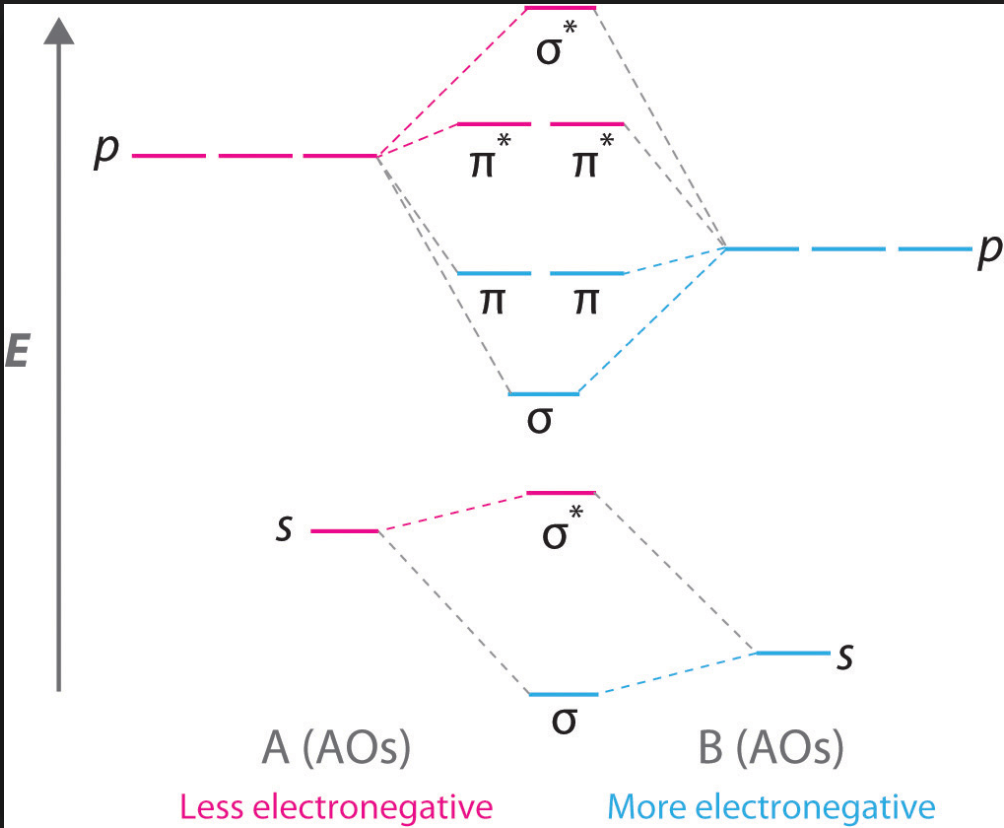
What are atoms with 7 or less protons?
When determining the pressure of gas that we had collected over water, we have to take into consideration this partial pressure.
What is the vapor pressure of water?
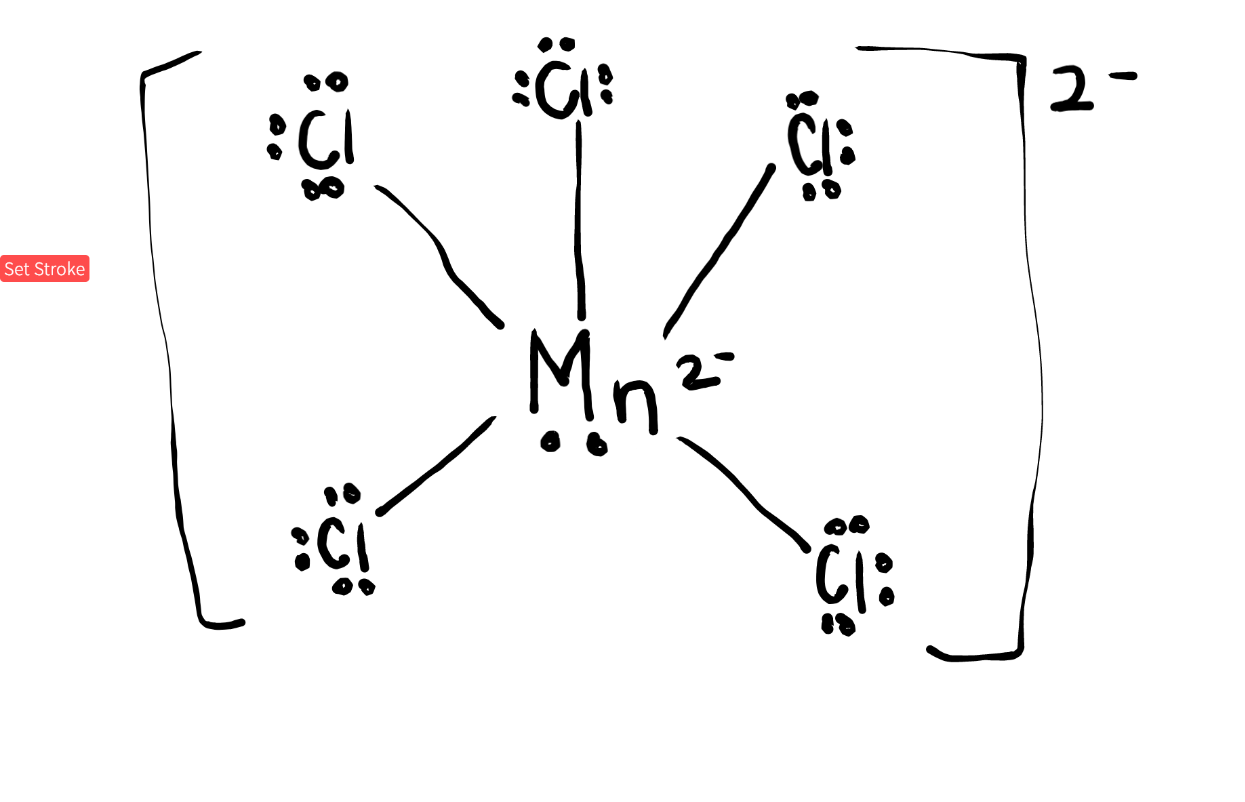
Draw and decide the EG, MG, bond angle, and hybridization of MnCl52-. (Double Jeopardy: Doesn't need to be in the form of a question)
Octahedral, square pyramidal, 90oC, sp3d2
In an episode of the hit show, Rick and Morty, Rick and Summer throw a party. During the party, the Smith house is transported to another dimension. In this dimension there is a chemical that forms Kalaxian Crystals (K-lax). The molecules in pure K-lax have a remarkable geometry unseen in our dimension with six bonding domains and two lone pair domains. What do you expect the hybridization to be assuming this dimension follows similar atomic rules as ours? (Doesn't need to be a question)
sp3d4
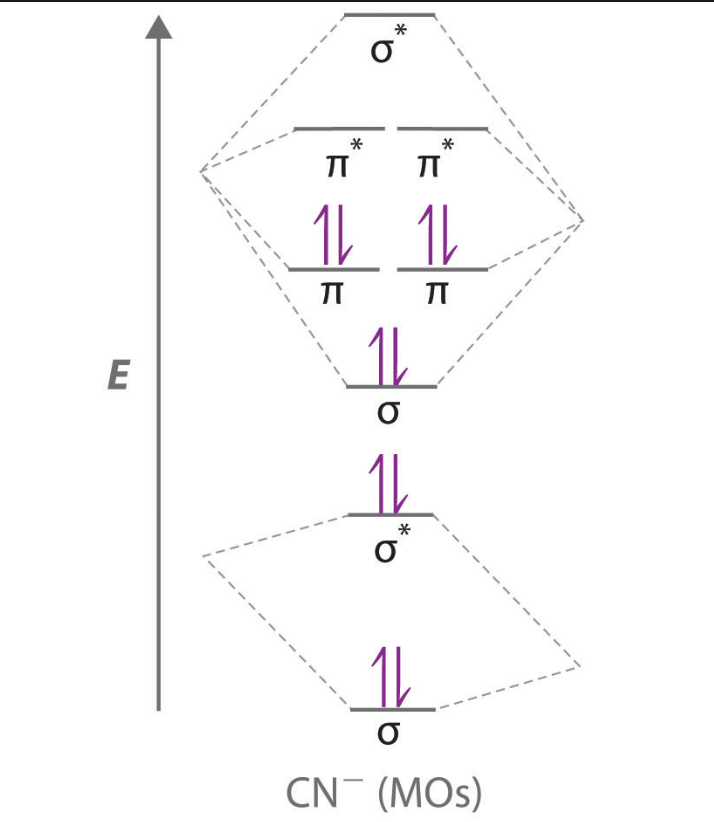
Draw the molecular orbital diagram for CN-. What is the bond order? How many sigma and pi bonds do you expect?
Bond Order=3.0, 2 pi and 1 sigma
This is the gas law for a real gas (aka Van der Waals Equation):
(P+(an^2)/V^2)(V-nb)=nRT
In this equation, what is 'a' and what is 'b'? (Does not need to be in the form of a question)
a=a constant who's value depends on the gas
b=the volume occupied my a particle of that gas per mole of that gas