This is the definition of a dynamic equilibrium.
What does it mean when a reaction is going both ways without any discernible change?
These are the 6 strong bases.
What are LiOH, KOH, NaOH, Ca(OH)2, Sr(OH)2, and Ba(OH)2?
Arrange the following in terms of increasing acidic strength:
ClCH2CH2CH2COOH, CH2CH2CHClCOOH,
CH2CH2CHBrCOOH, CH2CH2CH2COOH
CH2CH2CH2COOH<ClCH2CH2CH2COOH<
CH2CH2CHBrCOOH<CH2CH2CHClCOOH
If you heat up an exothermic reaction, this will happen according to Le Chatlier's principle.
Why did the reaction shift left towards reactants?
*In an exothermic reaction, heat is a product.
What is the difference between the Arrhenius definitions for acids and bases and the Bronsted-Lowry definitions?
Does not need to be in the form of a question
Arrhenius bases physically donate an OH- to solution. Bronsted-Lowry bases accept hydrogens.
For a triprotic acid Ka1 is expected to be the biggest followed by Ka2 and Ka3. Which Ka do you expect the biggest Kb to be inversely proportional?
Since, Ka3 is the smallest, that means this step's Kb is the biggest.
To determine if a concentration is at equilibrium, you will calculate this.
What is the equilibrium quotient, Q?
Ka and Kb describe two different reactions, not reverse reactions.
(What does this mean in terms of their relationship)
Why aren't Ka and Kb reverse reactions of each other?
*Reason we must use use Kw for the conversion
Delineate the successive deprotonation steps for oxalic acid: H2C2O4.
Does not need to be in the form of the question.
1. H2C2O4(aq) + H2O(l) <-> HC2O4-(aq) + H3O+(aq)
2. HC2O4-(aq) + H2O(l) <-> C2O42-(aq) + H3O+(aq)
This decreases due to the common ion effect.
Bonus for Double Jeopardy:
How is this explained by Le Chatlier's Principle?
What is molar solubility?
Bonus:
You are technically increasing the concentration of one of the products. According to Le Chatlier's principle this will cause a leftward shift, meaning less solid can dissolve in solution, therefore decreasing molar solubility.
How does percent ionization explain why we don't use ICE tables for strong acids/bases?
Does not need to be in the form of a question
Since strong acids and bases ionize pretty much 100%, this means there is no equilibrium for and ICE to describe. The reaction just goes to completion.
Will the following be more soluble in an acid solution or a basic solution?
BeCl2Does not need to be in the form of a question
In a basic solution.
*Be2+ is a week acid with a weak conjugate base.
Find K for 2N2O(g) + 3O2(g) <-> 2N2O4(g) using the following:
1) 2N2(g) + O2(g) <-> 2N2O(g) K1=1.2x10-35
2) N2O4(g) <-> 2NO2(g) K2=4.6x10-3
3) 1/2N2(g) + O2(g) <-> NO2(g) K3=4.1x10-9
K=1.1x106
Convert this pH to pOH using the Kw method.
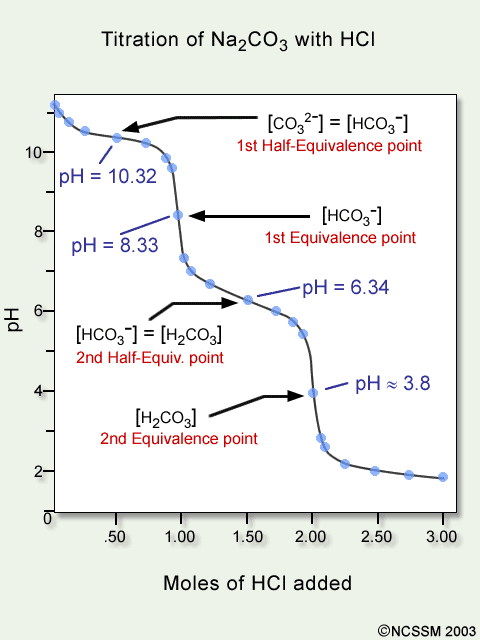
pH=4.1Draw and label the titration curve of a diprotic base.