CO has what molecular shape?
Only 2 atoms always means linear
CO
carbon monoxide
Draw CH4
H
|
H - C - H
|
H
prefix for 9
nona
Define a covalent compounds
Draw water, what's its molecular shape?
Bent as the central atom (O) has 2 lone pairs and is connected to 2 atoms
H2O
dihyride monoxide
Draw a diagram of CH2O
..O..
||
H - C - H
How many valence electrons does S have
6 because its in group 16
Why are some atoms only in compounds of paired with another atom of the same element?
Because it's not stable alone!
What molecular shape is this?
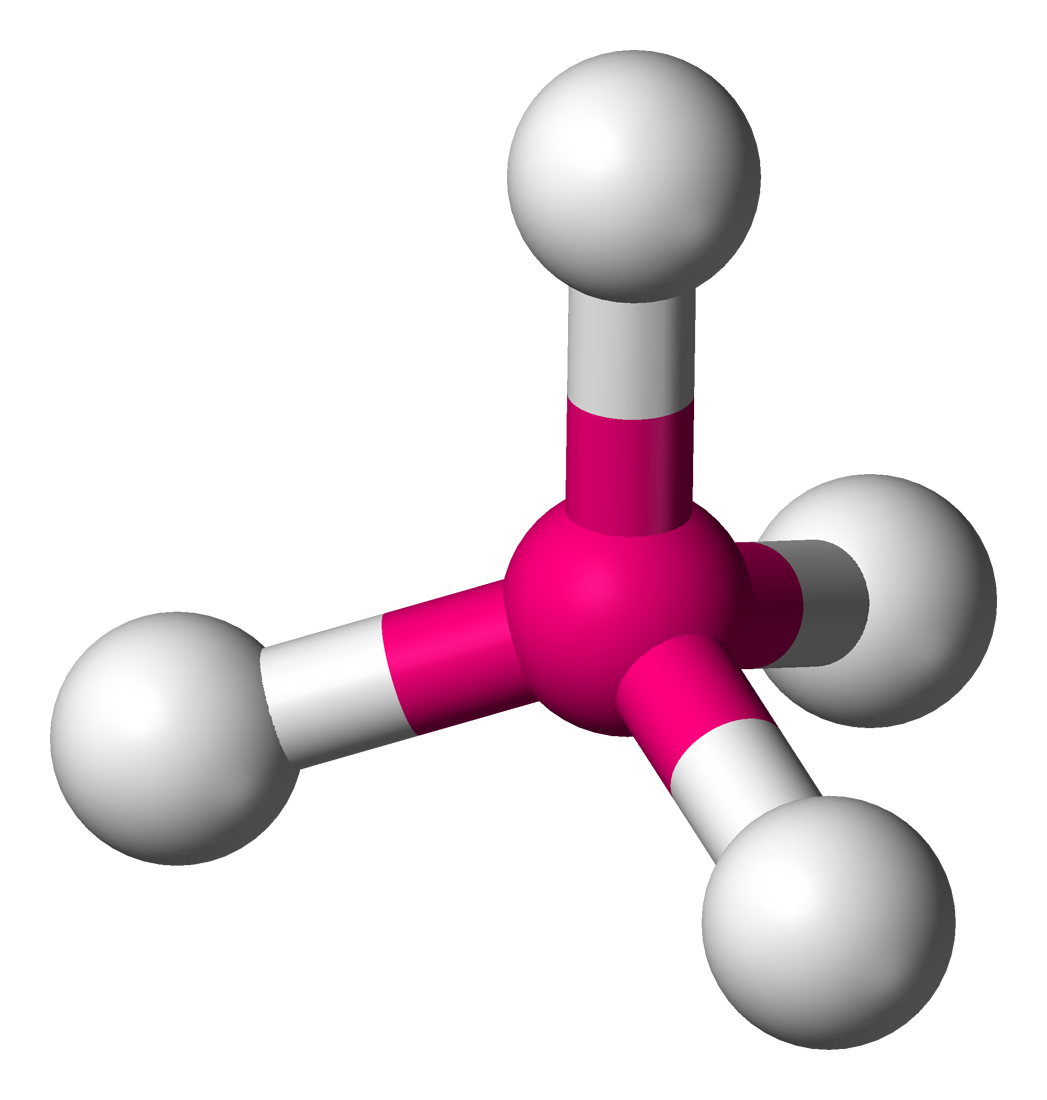
Tetrahedral
5 total atoms, central atom connected to 4 atoms with no lone pairs
C6H8
hexacarbon octahydride
Draw a line structure of CO2
. . . .
O = C = O
. . . .
The atom we look at to determine molecular shape
central atom
Define a covalent bond
Sharing of electrons
Draw a line structure of PH3 to find the molecular shape
Trigonal pyramidal
The central atom is connected to 3 atoms AND has a lone pair of electrons
Si7F
heptasilicon monofluoride
Draw a line structure of NH3
. .
H - N - H
|
H
How many bonds does phosphorus want to make?
3 because its in group 15
Number of lone pairs on the central atom in one molecule of H2S
two lone pairs
Draw a line structure of SF6 to help you find its molecular shape
Trigonal bipyramidal because the central atom (S) is connected to 6 atoms.
C5P9
pentacarbon nonaphosphide
Draw a line structure of CH3SH (Hint: the fourth H doesn't go on my central atom)
H
|
H - C - H
|
S - H
Lithium has 1 valence electron. What would this atom do to create a full octet?
Lose 1 electron to reach a noble gas electron configuration
I2 and H2 are example of
diatomic atoms