Week 1 Physical Change and Etc
Week 1 Sig Figs and Dimensional Analysis
Week 2 Atomic numbers and electrons
Week 2 Laws and Etc
100
Define physical change as well as chemical change.
a physical change is a change in properties such as texture, shape, or state, while a chemical change represents the formation of a new substance after atoms are rearranged in a chemical reaction.
100

3
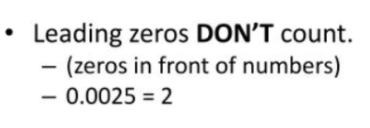
100

62
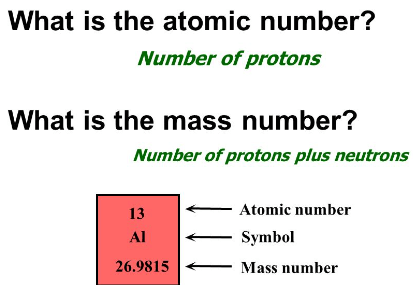
100

Protons orbit around the nucleus of the atom
*Electrons are what actually orbit the nucleus
200

5.967 mL
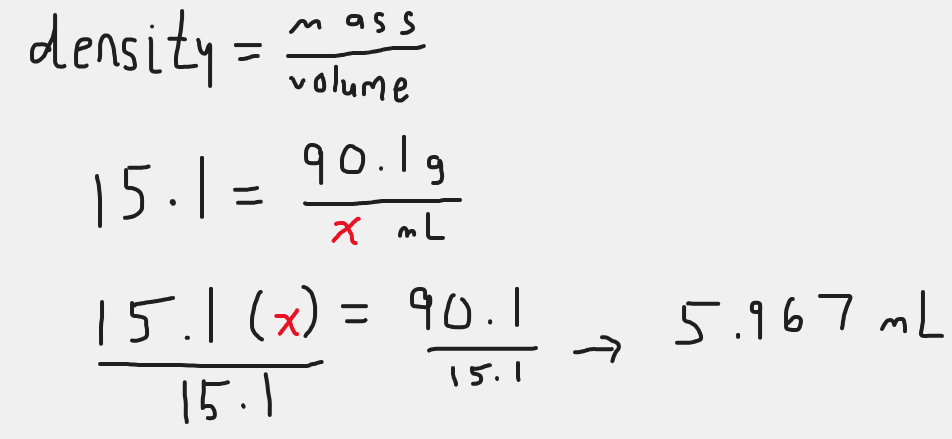
200
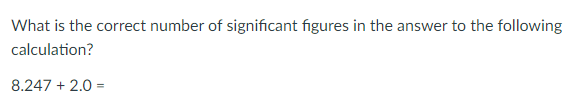
3 sig figs
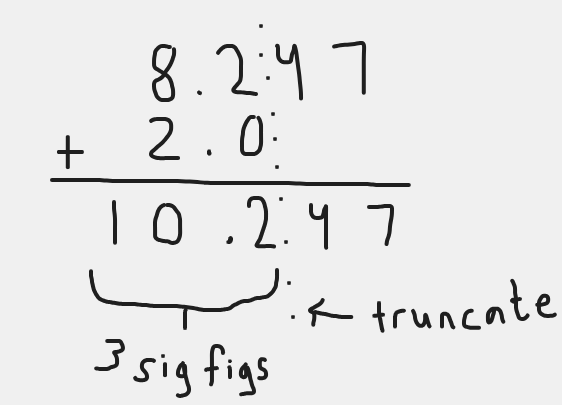
200

18
*A neutral Ca atom would have 20 electrons but the +2 would remove 2 electrons making 18 e-
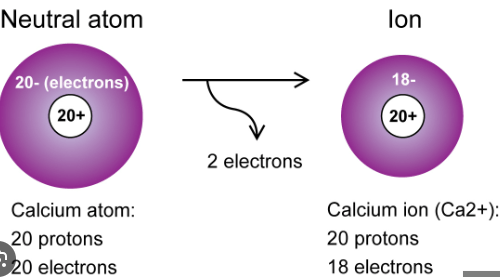
200

1.68%
300

fireworks exploding
300
Convert 0.618 g to cg
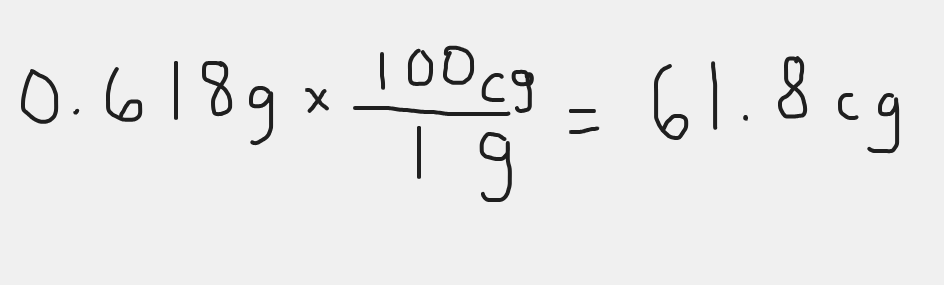
300
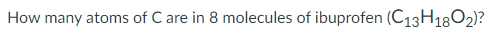
104 Carbon atoms
*take 8 x 13
300

118.944 amu
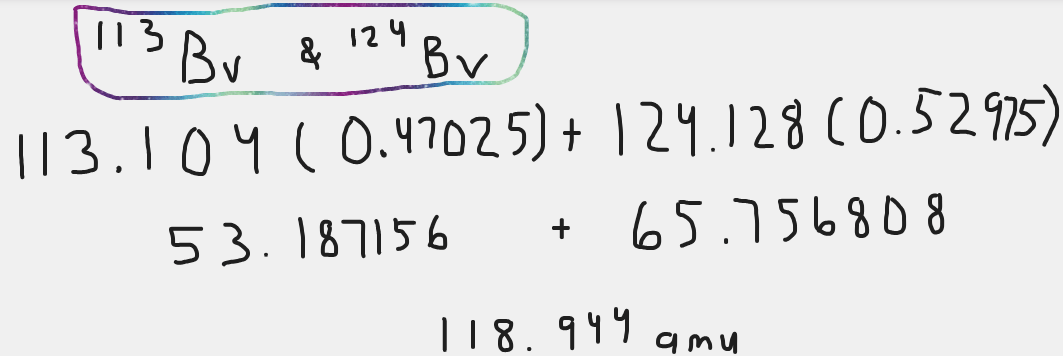
400

weight
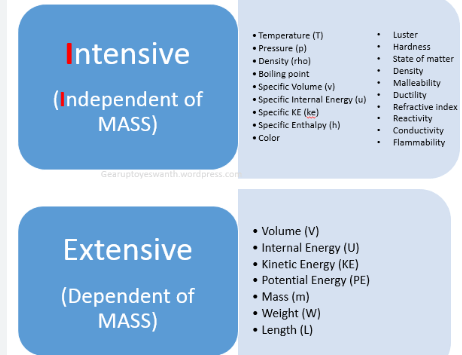
400
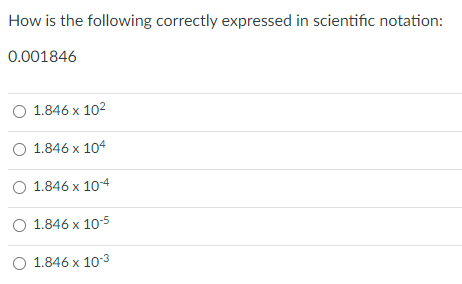
1.846 x10-3
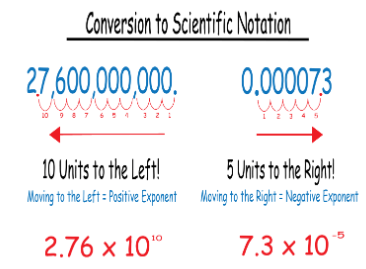
400

6 Hydrogens
400

11.54 Y