Warm-up
Warm-up
Explain in your own words what the Bronsted-Lowry explanation of Acids and Bases is.
acid= proton (H+)donor
base= proton (H+) acceptor
H2O
* remember: the difference between acids and bases is 1 proton (same as H+) so to find OH- 's conjugate acid you would add a H+
*another thing to remember is that H2O can act as an acid of a base.
An acid or base is considered strong because of the degree of what?
the degree to which it ionizes. It is a strong base or acid if it ionizes completely.
What is the [H3O+] in a solution with a pH of 8.66?
2.2 × 10-9 M
*10-pH is how you would set this up
Conjugate acid-base pairs differ by only what one thing?
one H+
*an example is this diagram below that shows each pair and their conjugate acid and base. The acid has the H+, and the base is the same minus the H+
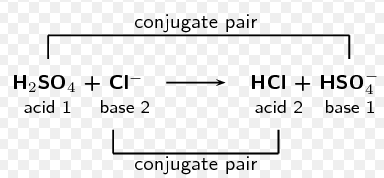
H2SO4 is an acid that has what conjugate base?
HSO4-
*to get this answer I removed an H+ from the original acid
A large value of Ka indicates what about an acid?
A large Ka value for an acid means that that acid is a strong acid
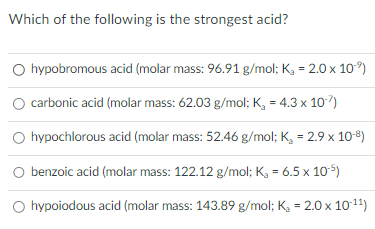
D) benzoic acid
*this is correct because benzoic acid has the highest Ka value.
If there is an acid on the reactant side, the conjugate base will be located on which side: the reactant side or the product side?
The product side
*Conjugate acid and base pairs will always be on opposite sides of a reaction.
In this forward reaction, is H2O acting as an acid of a base
NH4+ + H2O ⇌ NH3 + H3O+
H2O is acting as a base
*You can tell this by identifying it's conjugate acid, which is H3O+. We know H3O+ is the acid because it gained the H+ and H2O is the base because it lacks the H+
Out of these 3 acids, which one is the strongest?

HClO3
*explanation: The more oxygens added means that there is more distance away from other atoms causing the Hydrogen to be donated easier. The easier it is to donate a Hydrogen then the stronger the acid
What is the pH of a 7.2 x 10-5 M solution of HBr
pH= 4.14
*First determine 2 things: is it an acid or a base & is it strong or weak
HBr is an acid, HBr is also a strong acid (strong acids need to be memorized)
-Then determine pH from concentration 7.2 x 10-5 equation (pH=-log[H3O+])
Compare these acids and determine which is the strongest

Cl
* the more electronegative the middle molecule=stronger acid
Cl is farther up which means more electronegative
Out of these 3 acids, which one is the weakest?

HF
*the weaker the bond between Hydrogen and middle atom the stronger the acid. bond strength decreases down a group so HBr is the strongest and HF is the weakest