This state of matter has a definite volume and a definite shape.
Solid
What is the name of the process for when a liquid turns into a gas?
evaporation
Sophia is making a pizza. To do so, she must mix the flour, water, sugar, and yeast. Then she must place the mixture in a covered bowl until bubbles form and the dough rises. Is this a chemical or a physical change.
Chemical!
True or False: The substances of a solution are evenly mixed.
True
A change that produces a new substance.
Chemical Change
This state of matter has a definite volume but takes the shape of its container.
Liquid
What happens during the process of condensation?
A gas turns into a liquid!
Sophia is making a pizza. She takes her door and rolls it flat so that she can put toppings on it. Is this a chemical or a physical change?
Physical!
Harry places three pieces of ice in a glass of water. Is this a mixture or a solution?
Mixture
Mass cannot be created, nor destroyed. It can only be changed.
The Law of Conservation of Matter/Mass
This state of matter has no definite volume and fits the shape of any container.
Gas
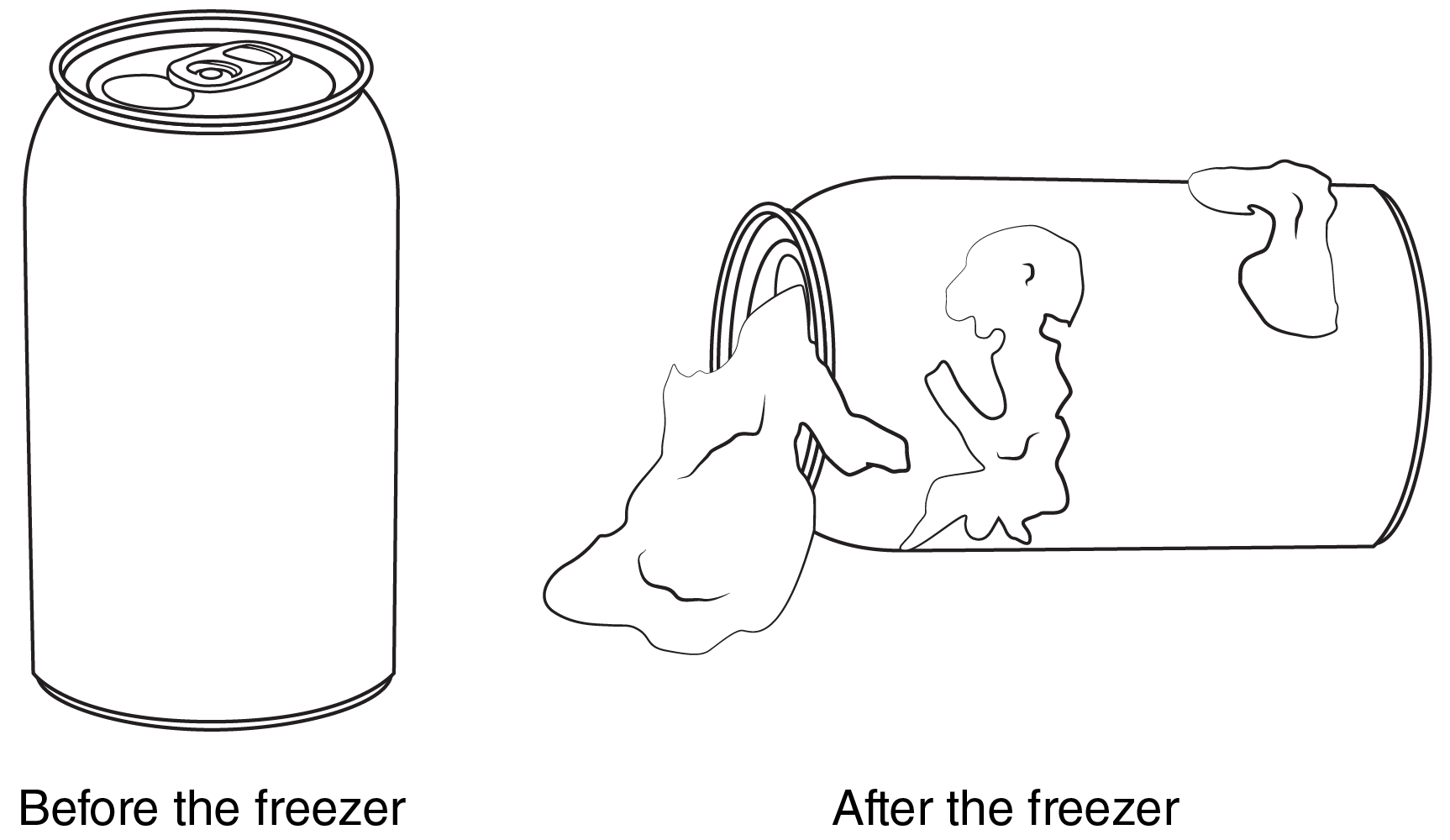
Emma placed a canned liquid drink in a freezer. She removed the can 12 hours later. The pictures show the can before and after Emma put it in the freezer. How can we describe what happened to the liquid?
It froze!
Sophia is making a pizza. When the pizza leaves the oven, the dough has browned and is hot to the touch. Is this a physical or a chemical change?
Chemical!
Miss Albert mixes two substances together. The first substance weighs 8g. The second substance weighs 14g. What is the total mass of her mixture?
22 grams!
A substance where different materials are put together, but each keeps its own properties.
Mixture
Keisha’s teacher placed a substance in a jar for Keisha to observe. The teacher labeled the jar substance X. These are Keisha's observations.
Observations of Substance X
- clear color
- filled half of the container
- definite volume
- flowed to the other side when it was turned upside down
What state of matter is the substance that Keisha is observing?
Liquid!
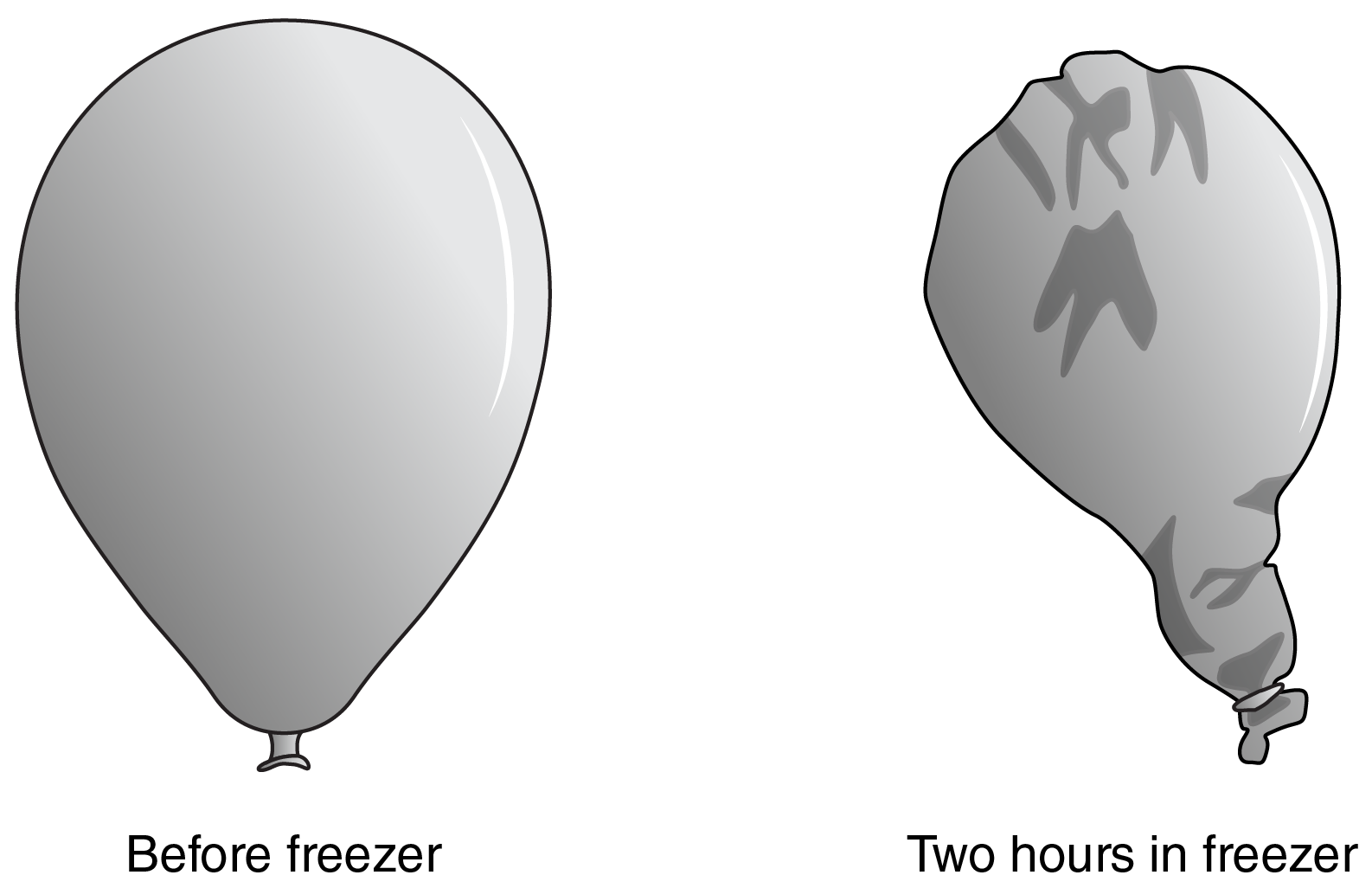
This balloon has been put into the freezer. What happened to the particles of the balloon?
The cold air caused the particles to compact or change volume!
Sophia repeats the experiment with two new substances, W and Z. She measures each substance and then mixes them together. When Sophia combines the two substances, they start to bubble. Once the bubbling stops, she measures the mass of substance WZ and finds that the mass is less than before she had mixed the two substances together. Why is this?
The reaction created a gas, which escaped into the atmosphere!
A group of students placed iron filings and sand in a jar. One student put the lid on the jar. Another student shook the jar.
One student opens the jar and pours it into a pan. Another student holds a magnet over the sand and iron filings. What is the purpose of this procedure?
To separate the substances of a mixture!
A gas turns into a solid.
Deposition
What would you observe if you poured a liquid from a small beaker into a larger beaker?
The liquid would take the shape of the larger beaker!
Lamar builds a robot out of blocks. He measures the mass of the robot on a scale. Lamar accidently drops the robot, and the robot breaks into pieces.
What can Lamar expect to find when he measures the mass of the broken pieces?
The mass stays the same!
What are the five signs that a chemical change has occurred?
1. Color Change
2. Odor
3. Gas is formed (bubbles)
4. Solid is formed
5. Heat is released!
Ben’s aunt mixes some sugar into her hot tea. She stirs the tea until she can no longer see any sugar particles. Did Ben's aunt create a new substance?
No
A mixture in which the substances are evenly spread out and do not settle to the bottom of the container.
Solution