What is a physical change
alters a substance's form (shape, texture or size), appearance, or state (like solid, liquid, gas) but not its chemical composition
What is the definition of a chemical change?
Answers: When atoms are rearranged, when a new substance is created, when atoms are breaking down or coming together.
Casey remembers that molecular motion increases as temperature increases. Identify what effect increased molecular motion.
A. Increasing the molecular motion reduces the solubility.
B. Increasing the molecular motion decreases the reaction rate
C. Increasing the molecular motion decreases the reaction rate
D. Increasing the molecular motion increases collision frequency
D. Increasing the molecular motion increases collision frequency
A change in matter that alters the shape, size, or state of a substance, but does not create a new substance.
What is Physical change
Compare the mass of an ice cube to the mass of the water once the cube is completely melted.
A. The mass of water formed equals the mass of ice
B. The mass of water formed is more than the mass of ice.
C. The mass of water formed is less than the mass of ice.
D. The mass of water formed does not depend on the mass of ice.
A. The mass of water formed equals the mass of ice.

Describe the relationship of the atoms shown above.
Responses
- A. The rearrangement of atoms is a chemical change.
- B. Stability of the atoms in the molecule means a physical change.
- C. A change in state of matter from a liquid to gas is a physical change.
- D. A change in state of matter from solid to liquid is a chemical change.
C. A change in state of matter from a liquid to gas is a physical change.
Water freezing or turning into ice
Color change always indicates chemical change
True or False?
what is False
Ms. Collins did an experiment with baking soda and vinegar. She started out with 45g of matter and after the experiment she was left with the same amount. What system did she use and why? 
closed system; the weight stayed the same because no matter escaped
What is this an example of and why?

Physical change; popcorn is only changing apperance or shape
Judging by these atoms, which one is most likley in exposed to the most heat?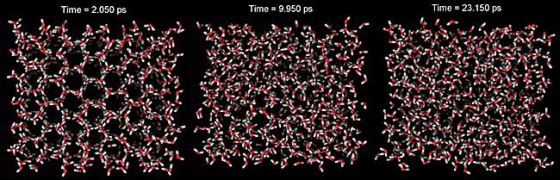
#1 #2 #3
What is #3
Identify which property changes as temperature increases to increase the reaction rate.
Responses
- A. Increasing the temperature decreases the molecular motion.
- B. Increasing the temperature reduces the collision frequency.
- C. Increasing the temperature divides the solubility of the solute.
- D. Increasing the temperature increases the energy of the molecules.
- D. Increasing the temperature increases the energy of the molecules.
A plant undergoing photosynthesis. Physical or chemical change?

Chemical change; 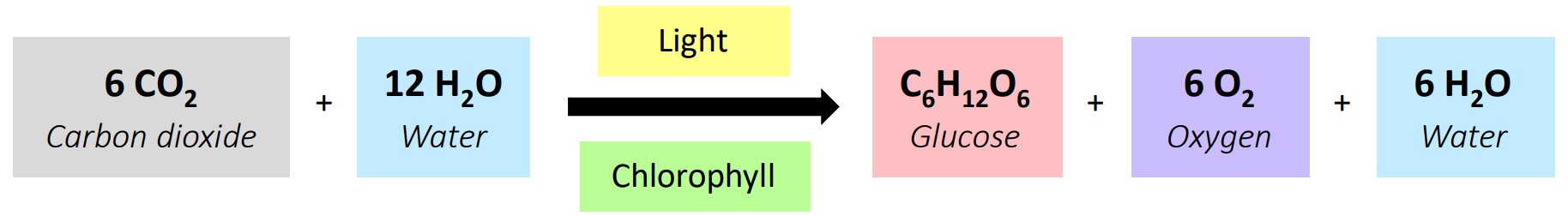
Elaborate on the difference between a chemical change and a physical change.
Physical change does not break down bonds
Chemical change forms new substances/atoms rearrange
Judging by these atoms, which one is a solid?

#1 #2 #3
What is #1
The smell of rotten eggs forming from an egg left out too long is a sign of this kind of change
chemical change
NaOH + HCl → NaCl + H₂O
16 g 24 g 12 g _?_
*hint:use algebra
1. 16+ 24 = 40g
2. 40g-12g=
Answer: 28g
When baking a cake, this type of change happens because new substances form and it cannot be reversed back into original ingredients./images.kitchenstories.io/articleContentImages/RP16_04_32_CONTENT2_cakebaking101_04_85854900.jpg)
What is Chemical change
When the temperature of a chemical reaction is increased, this generally happens to the rate of the reaction because the particles move faster and collide more often.
What is rate of reaction increases
Name three common indicators scientists use to identify that a chemical change has occurred
2. Heat
3. drastic color change