How many carbons (C) are in CO2
1
What is kinetic energy?
The energy of motion
What type of change is chemical reaction that forms new products?
Chemical Change
What is the name of this element?

Neon
What are the reactants for this reaction: CH4 + O2 -> CO2 + H2O?
CH4 + O2
How many oxygens (O) are in H2PO4
4
What happens to kinetic energy when thermal energy is increased?
Kinetic energy increases
What type of change is combustion?
Chemical Change
What is the symbol for this element

Li
What are the products for this reaction: CH4 + O2 -> CO2 + H2O?
CO2 + H2O
How many oxygens (O) are in 4O2
What happens to the attraction between particles as thermal energy increases?
The attraction between particles decreases
What type of change is shredding a piece of paper?
Physical Change
What is the atomic number for this element?

11
How many Oxygens (O) are on the reactant side of this reaction:
2NaOH + H2SO4 -> Na2SO4 + 2H2O ?
6 Oxygens
How many hydrogens (H) are in 4Ca(OH)2?
8
What state of matter has a fixed shape and a fixed volume?
Solid
What type of change is melting an ice cube?
Physical Change
What is the atomic mass of this element?
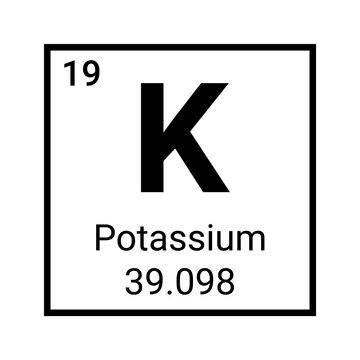
39.098
How many Oxygens (O) are on the product side of this reaction:
CaCO3 -> CaO + CO2 ?
3 Oxygens
How many sulfurs (S) are in 4Cu(HSO3)2
8
Liquid
What type of change is boiling water?
Physical Change
What is the number of protons in this element?

77
How many chlorines (Cl) are there on the product side in this reaction:
FeCl3 + 3H2O -> Fe(OH)3 + 3HCl
3 Chlorines