Na
What is sodium?
a force that holds two or more atoms together
What is a Chemical Bond?
What kind of chemical bond is formed when an equal exchange of electrons occurs
What is Ionic?
What is the total number of atoms in the compound Ca(ClO3)2
What is 9?
Ca
What is Calcium?
a group of atoms held together by covalent bonding that acts as an independent unit
What is a Molecule?
What occurs between atoms that do not easily lose electrons
What is Covalent Bonding?
In a chemical formula, the number of each type of atom in the compound is shown by numbers are called . . .
What are subscripts?
H
What is Hydrogen?
an atom that is longer electrically neutral because it has lost or gained valence electrons
What is an Ion?
What do atoms gain, lose, or share when they bond
What are Electrons?
Why do the noble gases NOT form compounds readily
What is because their outer energy levels are completely filled with electrons?
S
the attraction between positively and negatively charged ions is an ionic compound
What is an Ionic Bond?
How many electrons fill the outermost energy level of an atom
What is 8?
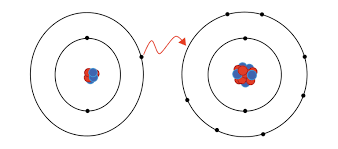
What is Ionic Bonding?
Cu
What is Copper?
a bond formed when many metal atoms share their pooled valence electrons
What is a metallic Bond?
What is the joining of atoms to form new substances?
What is chemical bonding?
