Thermodynamics is applied to learn a reaction's _____.
Kinetics is the _____ of a process.
feasibility
speed
review sheet, question 1
15.04
What is the meaning of spontaneous processes?
Physical or chemical change that occurs by itself
15.03
Which of the following is equal to the change of heat at constant pressure?
a. TtriangleS
b. triangleH
c. triangleG
d. triangleU
e. triangleS
b. triangleH
review sheet, question 4
What are the three molecular motions related to entropy?
stretching, asymmetric stretching, and bending
15.15
triangleG=0 the system is at _____
equilibrium
15.50
The total entropy of a system and its surroundings always increases for a spontaneous process. This is a statement of...?
The second law of thermodynamics
review sheet, question 8
Most spontaneous processes are _____.
exothermic
15.07
Which of the following has a positive value for an endothermic reaction?
a. free energy change
b. equilibrium constant
c. entropy change
d. enthalpy
e. enthalpy change
e. enthalpy change
review sheet, question 9
The _____ dispersed the energy, the higher the entropy.
The _____ places you can put energy, the higher the entropy.
The _____ ways you can arrange a system, the higher the entropy.
more
15.08
triangleG<0 _____ in forward direction
spontaneous
15.50
A ______ process releases energy to surroundings.
spontaneous
15.06
Which of the following is a/are spontaneous process(es)?
1. ice melting at -5^oC
2. heat flowing from a cold object to a hot object
3. an iron tool rusting in the presence of oxygen
3. an iron tool rusting in the presence of oxygen only
review sheet, question 2
Which of the following is/are true in the sublimation of one mole of dry ice?
1. entropy increases
2. entropy decreases
3. enthalpy increases
4. enthalpy decreases
1 and 3
review sheet, question 10
Entropy is an _____ _____ meaning it depends on the amount of substance present. It is also a ____ ____ meaning it depends on variables such as temperature and pressure, that determine the state of the substance.
extensive property
state function
15.12
triangleS_(rxn) ^o = sumnS_(?) ^o - summS_(?) ^o
products
reactants
15.47
What is the thermodynamic quantity that provides the criterion for the spontaneity of a chemical reaction?
a. TtriangleS
b. triangleH
c. triangleG
d. triangleU
e. triangleS
c. triangleG
review sheet, question 6
true or false: spontaneity can be affected by temperature.
true
15.29
-triangleH_(system) means the reaction is ___thermic and favors spontaneity.
triangleH_(system) means the reaction is ___thermic and works against spontaneity.
exo
endo
15.33
Which of the following reactions has the largest positive value of triangleS per mole of O2?
a. 2C(s)+O2(g)->2CO(g)
b. 2H2(g)+O2(g)->2H2O(g)
c. C(s)+O2(g)->CO2(g)
d. 2NO(g)+O2(g)->2NO2(g)
e. 2Mg(s)+O2(g)->2MgO(s)
a. 2C(s)+O2(g)->2CO(g)
review sheet, question 5
triangleG>0 ______ in the forward direction. Reverse reaction is ______.
nonspontaneous
spontaneous
15.50
The third law of thermodynamics states that the entropy of a perfect _____ substance is _____ at absolute _____.
crystalline
zero
zero
15.44
Name an example of a spontaneous process that is endothermic.
melting of ice in your palm
15.07
For the melting of ice as an example, only at temperatures above 273 K, ____ beats ____.
entropy
enthalpy
15.34
Which processes involve an increase in entropy?
1. Br2(l)->Br2(s)
2. (NH4)2Cr2O7(s)->N2(g)+4H2O(l)+Cr2O3(s)
3. 2KClO3(s)->2KCl(s)+3O2(g)
4. 2Hg(l)+O2(g)->2HgO(s)
2 and 3
review sheet, question 11
Calculate the standard entropy change for the following reaction at 25oC.
CaCO3(s) -> CaO(s)+CO2(g)
SoCaCO3=92.9 J/Kmol
SoCaO=39.8 J/Kmol
SoCO2=213.6 J/Kmol
160.5 J/Kmol
15.48
So _____ with molar mass and molecular complexity.
increases
15.13
A reaction must be spontaneous at all temperatures when ____ is negative and ____ is positive.
triangleH , triangleS
review sheet, question 3
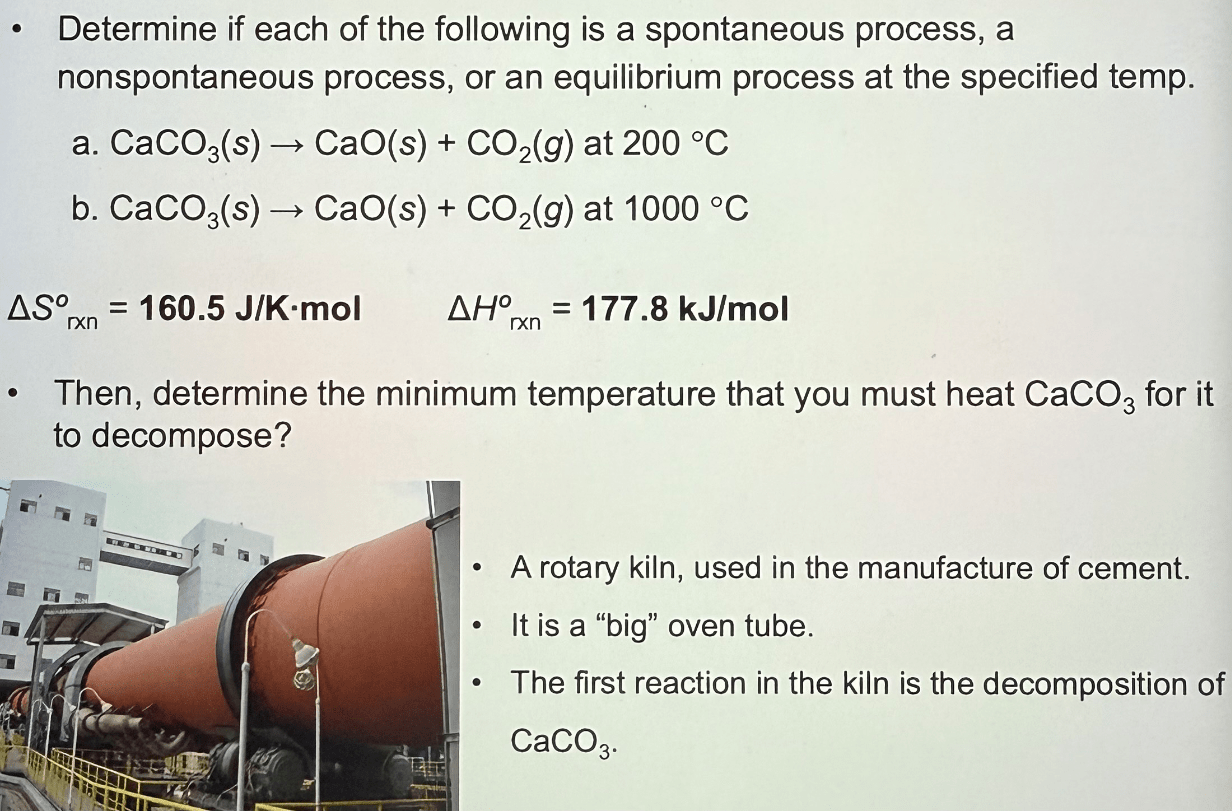
a. non spontaneous
b. spontaneous
835oC
15.43
Rank the following substances in order of increasing entropy at 298 K:
a. A2(g)
b. A2(l)
c. A3(g)
d. A2B(l)
b,d,a,c
review sheet, question 17
The standard free energy of formation is the ___ ___ change that occurs when __ mol of substance is formed from its elements in their standard states at 1 ___ and at 25oC.
free-energy
1
atm
15.58
What belongs in the two white boxes below?
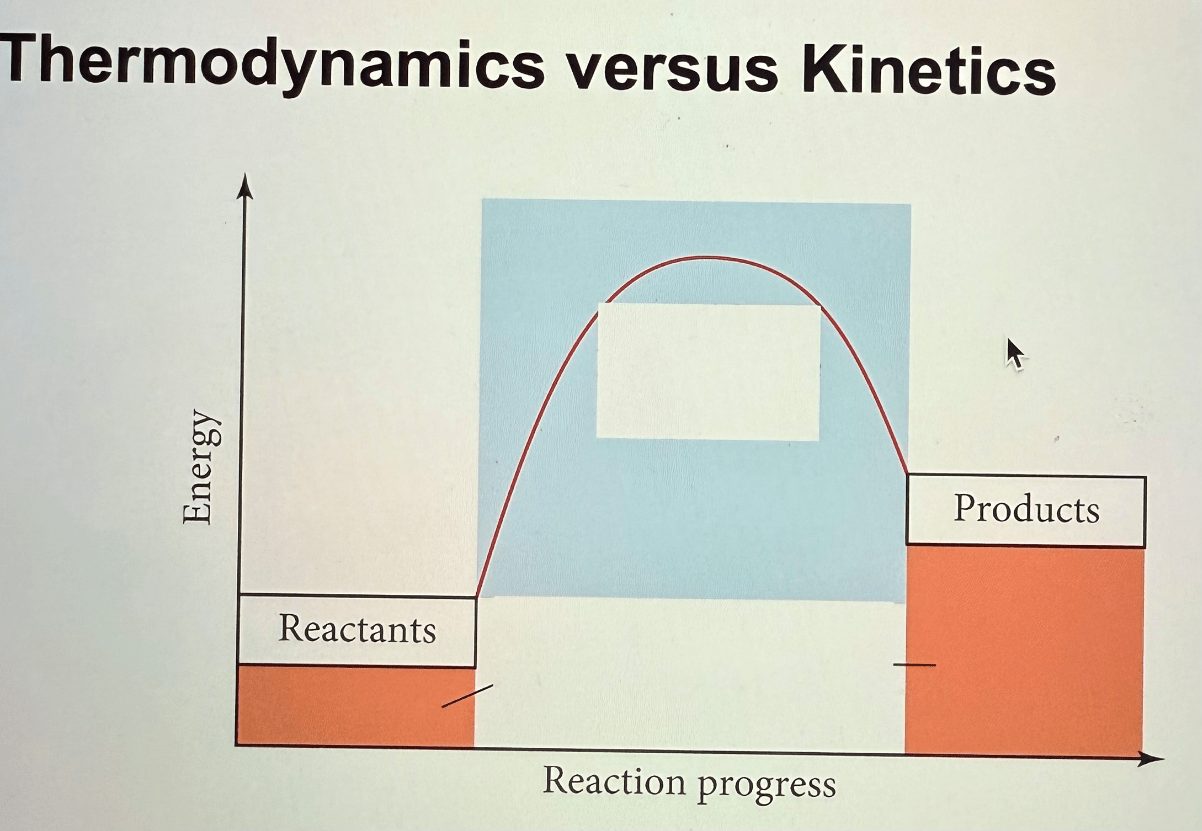
a. kinetics - intermediate states, speed
b. thermodynamics - initials and final states, spontaneity
15.04
1. triangleH_(sys) -TtriangleS_(sys) ? 0 reaction is spontaneous
2. triangleH_(sys) -TtriangleS_(sys) ? 0 reaction is non-spontaneous
3. triangleH_(sys) -TtriangleS_(sys) ? 0 reaction is at equilibrium
<
>
=
15.32
For a reaction in which triangleH=199.5 (kJ)/(mol) and triangleS=476J/Kmol , determine the temperature (in oC) above which the reaction is spontaneous.
146oC
15.41
2BCl3(g)+3H2(g) -> 2B(s)+6HCl(g)
Calculate the standard entropy of BCl3 if triangleS^o(rxn) = 161.8 J/molK.
Substance So(J/Kmol)
H2(g) 130.6
B(s) 5.9
HCl(g) 186.8
289.5 J/Kmol
review sheet, question 18
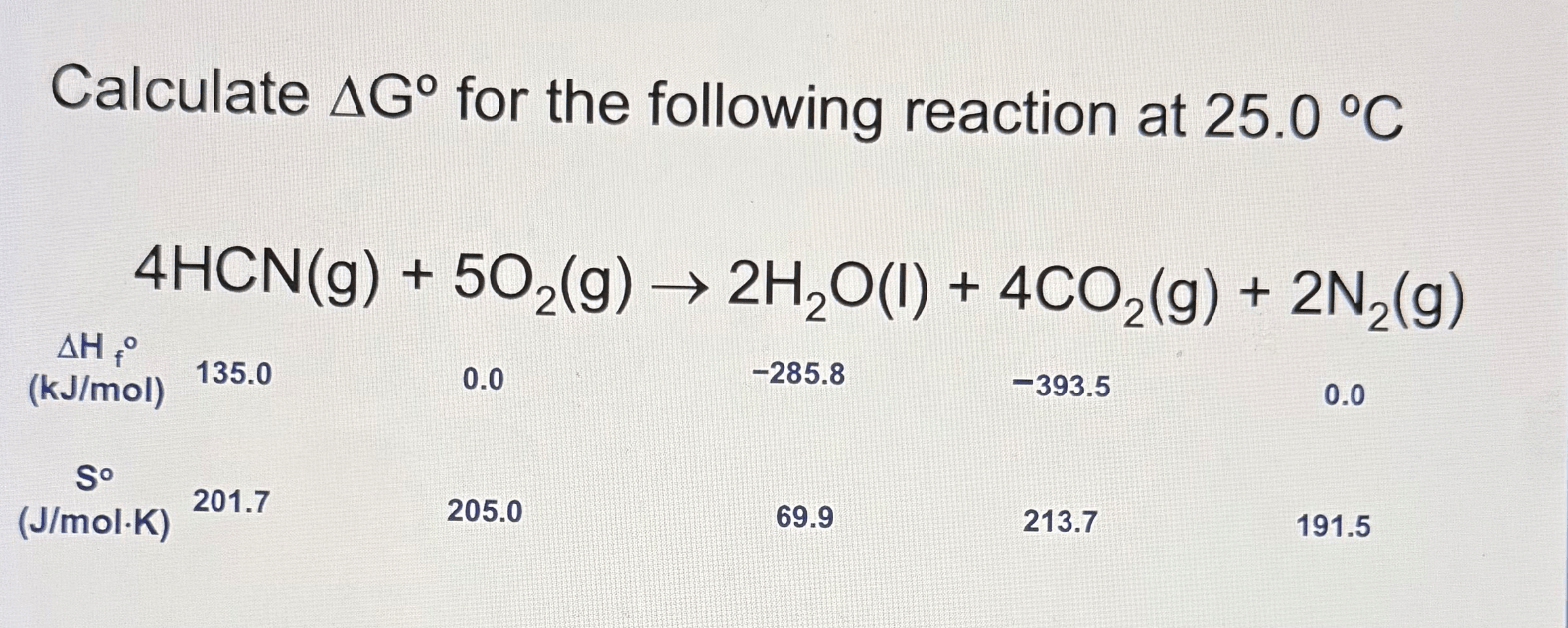
-2550. kJ/mol
15.56
What process is the conversion of diamond to graphite considered?
spontaneous
15.03
For a reaction in which triangleH =199.5 kJ/mol and triangleS =476 J/Kmol, determine the temperature (in oC) above which the reaction is spontaneous.
146oC
15.41
The entropy change of the process H2O(s) -> H2O(l) is 22 J/K at 0^oC . Calculate the enthalpy of freezing of water at 0^oC.
-6.0 kJ/mol
review sheet, question 19
HI has a normal boiling point of -35.4oC,and its triangleH_(vap) is 21.16 kJ/mol. What is the molar entropy of vaporization of HI, in J/molK?
89.0
review sheet, question 16
Calculate triangleG^o for the oxidation of ethyl alcohol to acetic acid.
CH3CH2OH(l)+O2(g) -> CH3COOH(l)+H2O(l)
triangleH^o =-495.2 kJ
triangleS^o=-136 J/K
T=298 K
-454.7 kJ
15.51