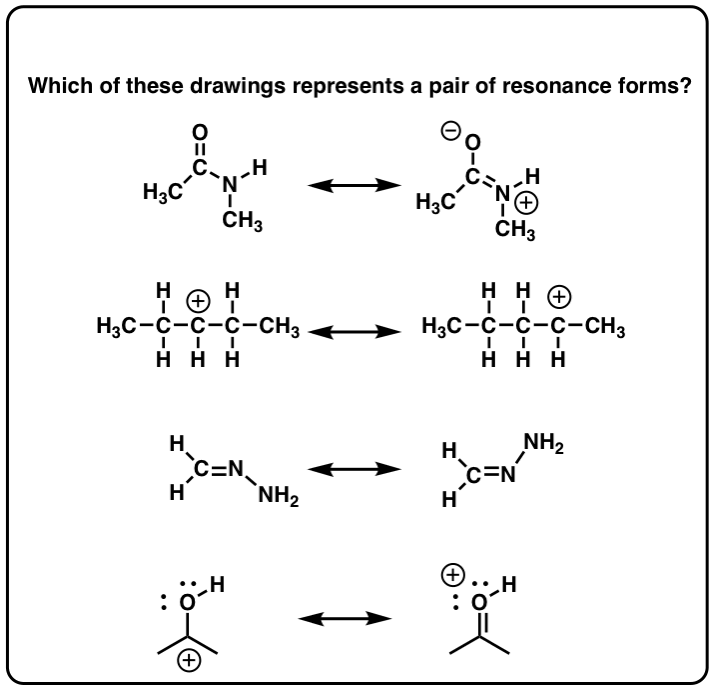
The last one
How many carbons in the molecule below:

6
what color is a lima bean
green
Draw an alcohol
If something has a steric number of 3, what is its geometry?
trigonal planar
Which one is the more stable resonance structure?
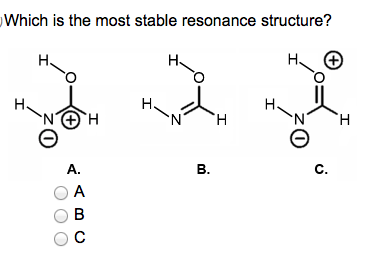
It is B, because that one has a neutral charge remember fewer charges are better and nitrogen likes 3 bonds O likes 2 bonds.
How many hydrogens are in the molecule below:

12
what is the shape of a kidney bean
kidney
Draw an alkyne
what is the hybridization of carbon in an alkane?
sp3
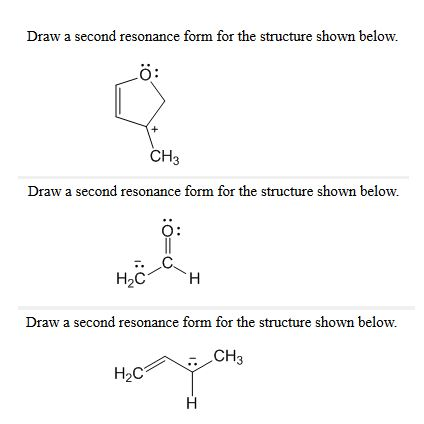
Draw a more stable resonance structure for the following:

Draw the bond line diagram of:
CH3CH2CH(CH2CH3)CH3
How many carbons are in the following molecule:

Draw an aromatic
what is the bond angle of a carbon in an alkene
120
Draw both of the resonance structures for the following:

One should have the carbocation in the cyclobutane and the other should have a carbonation that is on the end of the chain.
How many sigma bonds are in the following:

17
What type of bean is this:

Jelly bean
what is the hybridization of the carbon in an alkyne
sp
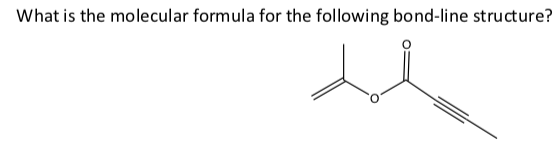
C7H8O2
0
Draw an amide vs an amine
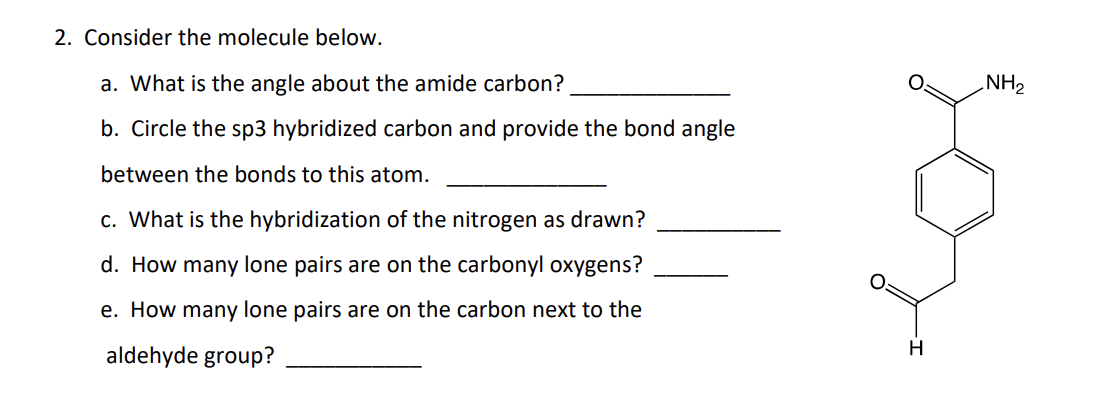
a. 120
b. carbon next to aldehyde carbon, bond angle is 109.5
c. sp2
d. 2
e. 0