Define: Model
A representation of a process or object in nature that allows us to more easily understand it.
An object more dense than water will _______.
Sink
T/F: An oreo weighs more than a goldfish. An Oreo must have more mass.
True
The nucleus of an atom contains these two particles.
Protons and neutrons
Chihuahua, St. Bernard, Boxer
Define: Matter
A general term for any physical matter
Adding electrons to an atom will give it a ___________ charge.
negative
What is thee charge of:
a) a proton
b) an electron
c) an neutron
a) positive
b) negative
c) no charge
Name this model:
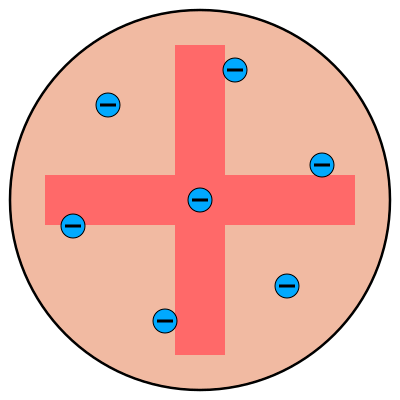
Plum-pudding model
brown, black, grizzly
Bear
Define: Mass
A m measure of how much matter exists in an object
The _________________ model states that an atom is a large positive charge with smaller negative charges scattered throughout it
TF: Atom A has 12 protons, 12 electrons, and 5 neutrons. Atom B has 12 protons, 12 electrons, and 10 neutrons. Atom B is an isotope of atom A.
True
List the 4 models that we studied in chronological order
Plum-Pudding Model, Planetary Model, Bohr Model, Quantum-Mechanical Model
blue, pilot, beluga
Whales
Define: Density
The mass-to-volume ratio of a substance
As electrons lose energy they travel _________ to the nucleus of the atom.
closer
Atom A has 11 protons and 7 neutrons. How many electrons does it have?
11 electrons
This model states that electrons can only orbit the nucleus of an atom on fixed orbits. They require a specific amount of energy to move farther away.
The Bohr Model
garter, milk, coral
Snakes
Define: Centripetal force
A force that acts on an object moving in a circle, directed toward the center of the circle
The _______ Model states that electrons orbit the nucleus in specific orbits.
Bohr
What did the Franck-Hertz experiment show?
That atoms can only accept specific amounts of energy.
Which model uses orbitals to describe the path that an electron travels rather than orbits?
The quantum-mechanical model
King, Dungeness, Tanner
Crabs
Define: Mass spectrograph
An instrument that measures the mass of individual atoms or molecules
Overlapping waves will result in larger or smaller waves. This is called an ____________ ______________.
Interference pattern
The electron clouds found in the quantum-mechanical model of an atom can also be referred to as this:
orbitals
This model is the currently accepted model of an atom
The quantum-mechanical model
European fallow, Mule, Sika
Deer