1. A compound that has a double bond between two carbon atoms is called an
What is an alkene
Which isomers can have different physical or chemical properties?
a. structural isomers only
b. geometric isomers only
c. both structural isomers and geometric isomers
d. neither structural isomers nor geometric isomers
What is c?
What is another term for cis-trans isomers?
What is geometric isomers
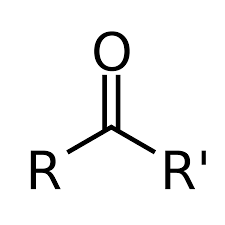
What is the structure of a carbonyl?
What is look at the board!!
What are hydrocarbons?
Compounds that contain hydrogen and carbon only.
2. What is the general molecular formula for alkanes?
What is CnH2n+2
A carbon atom with 4 different groups attached is termed a what
What is a chiral centre
a. addition reaction.
b. substitution reaction.
c. condensation reaction.
d. elimination reaction.
17. What is the general formula for a ketone?
If an alkane has 20 carbon atoms, how many hydrogen atoms will it have?
What is 42?
4. The straight-chain alkane with six carbon atoms is called
a. butane.
b. pentane.
c. hexane.
d. heptane.
What is c?
8. Alkenes can have two geometric isomers because
a. they are hydrocarbons.
b. they have a double bond.
c. they can form bonds with halogens.
d. they can be formed in elimination reactions.
What is b?
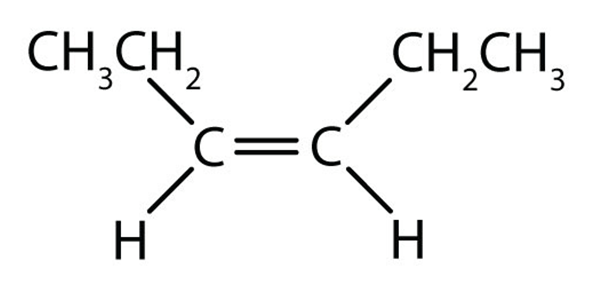
Draw cis-3-hexene
18. What is the general formula for an ester?
/ester-59134cd83df78c9283519859.png)
Which types of compounds are able to rotate plane polarized light?
What are optical isomers
3. The formula CH3CH2CH2CH2CHCH2 represents an
a. alkane.
b. alkyne.
c. alcohol.
d. alkene.
What is d?
Is cis-1,2-difluorocyclopentane chiral?
What is no
How many chiral centers does 2,3-dimethylpent-4-ene have?
What is 1
What is the word equation of a general combustion reaction
hydrocarbon + oxygen → carbon dioxide and water
What are the terms for compounds that rotate plane polarized light toward the right and the left?
What is (-) enantiomer (anticlockwise) and (+) enantiomer (clockwise)
5. The hydrocarbon, C6H12, is an example of a(n)
a. alkane.
b. cycloalkane.
c. alkyne.
d. aromatic.
What is b?
10. Butane and methylpropane are an example of a pair of
a. structural isomers.
b. geometric isomers.
c. positional isomers.
d. functional-group isomers.
What is a?
Common fuel for cooking and heating
What is propane
What is the balanced symbol equation of a methane combustion reaction
CH4 + 2O2 → CO2 + 2H2O
What is a racemate / racemic mixture?
What is a solution of equal amounts of two enantiomers so that the mixture is optically inactive.