Which parts of the atom have a negative charge and move around the nucleus?
electrons
Mendeleev created the first periodic table by arranging elements in order of
increasing atomic mass
Which group contains the most elements?
Metals
This is the most common element in the universe
Alpha and beta decay are almost always accompanied by
gamma radiation
What name is given to the part of the atom that contains the electrons?
electron cloud
Rows or columns all have similar characteristics?
Columns.
Rows have properties that change in a pattern.
A material is said to be ductile if it
Which group of elements have some properties of metals and some properties of nonmetals?
Metalloids
Alpha
Who discovered that that an atom’s positive charge must be packed in a tiny region in its center.
Ernest Rutherford
In the periodic table, the most reactive metals are found
Which elements are malleable, ductile, and can carry an electric current.
metals
Group 17 is so reactive that many of its elements are already in __________.
parts, substances, compounds, or properties
compounds
These radioactive isotopes can be followed through the steps of a chemical reaction.
tracers
Who discovered the electron?
J.J. Thomson
The atomic number of an element is based on the
number of protons in the nucleus.
Groups 3-12 are called the _____________ metals.
transition
Two of the same atoms bonded together is called a(n)
diatomic molecule
Looking at a Periodic Table, name an element (or Chemical Symbol) that has similar properties of Nickle (Ni).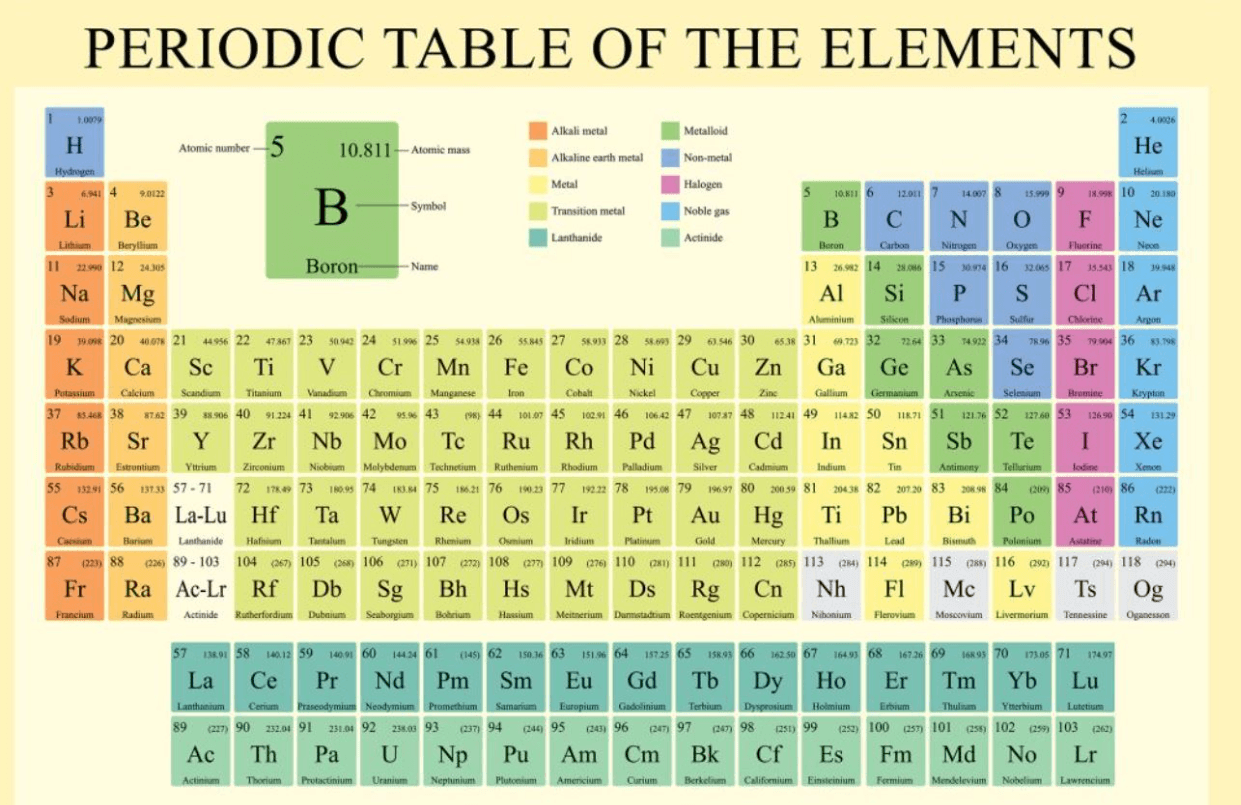
Pd Palladium or Pt Platinum or Ds Darmstadtium
(any in the same column)
This man’s model of the atom looked like a sphere or marble
Dalton
The elements that are unreactive are the
Noble gases
Alkaline Earth Metals
Halogens react to form_____________.
salts
If Carbon-14 decays to produce a beta particle, what element is created? (Beta Decay)
Nitrogen-14