This scientist proposed that atoms of elements could combine in whole number ratios to form compounds.
John Dalton
True or false:
Protons and electrons have opposite charges but the same mass.
False
In an ionic compound the positive and negative charges must be
equal
A cation has what charge?
Positive
Of the following elements which exists as a diatomic?
Helium, Sulfur, Iodine, Sodium, Tin
Iodine
J. J. Thomson discovered the electron using this famous experiment.
The cathode ray experiment
These two atoms are called
40/20Ca
39/20Ca
Isotopes
Atoms in group 13 on the periodic table form ions with this charge.
+3
An atom can form a cation by
Losing electrons
Name the element in period 5 on the periodic table that is an alkaline earth metal.
Strontium
This model of the atom is called
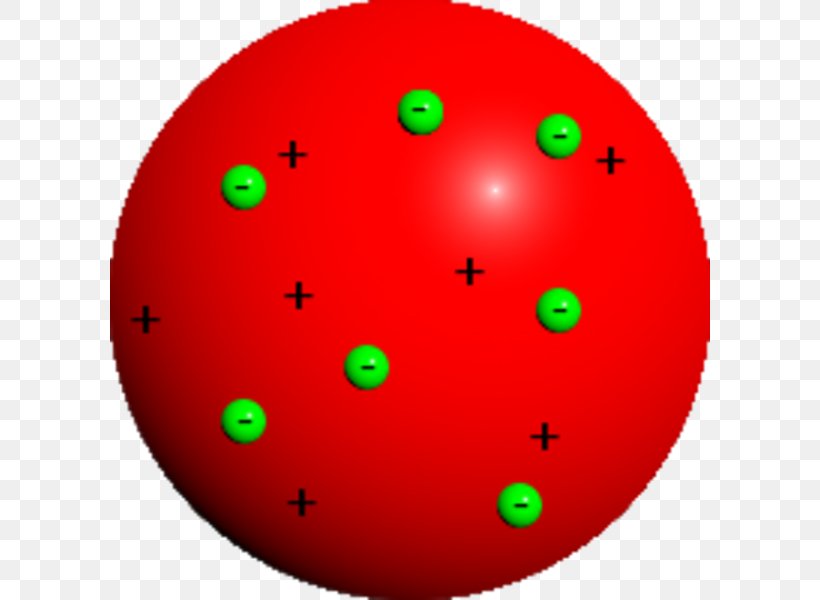
Plum pudding model
The function of neutrons in the nucleus is to
Hold the positively charged protons together
The formula of the compound formed between rubidium and iodine is
RbI
How many electrons does a sulfide ion contain?
18
The formula of the compound formed between sodium and nitrogen is
Na3N
In the gold foil experiment, Rutherford expected the alpha particles to
Go straight through the gold foil
Two important things are determined solely by the nucleus of an atom. What are they?
Its identity and its mass.
The formula of the compound formed between barium and phosphorus is
Ba3P2
Atoms in this group on the periodic table do not form any ions. Name the group.
Noble gases
Elements that border the staircase line on the periodic table are called
Metalloids
Prior to Democritus Greek philosophers thought that matter was composed of these four components.
Earth, water, fire, and air
The following atom has how many neutrons?
114/49 In
65
When an ionic compound dissolves in water its ions are free to move. This permits the solution to
Conduct electricity
Which of these atoms has 18 electrons?
20/17Cl^-
39/19K^+
40/18Ar
All have 18 electrons
A compound with the following formula contains how many oxygen atoms?
ZrO(NO3)2
7