Who discovered electrons using a cathode ray tube?
J J Thomson
It indicates the number of protons inside an atom
Atomic Number
Triple Points!!!
If Hg atomic number is 80, how many protons does the Isotope Hg-201 have?
80
What are the sublevels for electrons?
SPDF
How many valence electrons does bromine have?
7
Who said that electrons act like both particles and waves?
DeBroglie
Triple Points!!!
It indicates the sum of protons and neutrons inside an atom
Mass Number
If Mg atomic number is 12, how many electrons does the Isotope Mg-26 have?
12
Double Points!!!
How many orbitals are in each individual sublevel?
S = 1, P = 3, D = 5, F = 7
What is the electron dot notation for Ge?
Sr, Ca, Mg, Be
Double Points!!!
Who was the scientist that confirmed Democritus atomic model?
John Dalton
If an atom of Chlorine (Cl) has 17 protons and 20 neutrons, what is its mass number?
37
If I atomic number is 53, how many neutrons does the Isotope I-127 have?
74
How many electrons are in the 6th PEL?
18
What charge would oxygen have if it gained two electrons?
-2
Who was the first scientist to discover the existence of the nucleus inside the atom using the gold foil experiment?
Ernest Rutherford
If an atom of Nickel (Ni) has an atomic number of 28 and a mass number of 59, how many neutrons does it have?
31
What is the difference between mass number and atomic mass?
Mass number is the actual number of protons and neutrons in an atom. Atomic mass is the weighted average for all known isotopes of an element.
What is the electron configuration for Fe?
1s2 2s2 2p6 3s2 3p6 4s2 3d6
Double Points!!!
Al element has 13 protons, 14 neutrons, and 10 electrons. What is its charge?
+3
Who was the first scientist that stated electrons moved around the nucleus in orbits of fixed sizes and energies?
Niels Bohr
If an atom of Platinum (Pt) has an atomic number of 78 and a mass number of 195, how many neutrons does it have?
117
Bonus! Strontium (Sr) has four naturally occurring isotopes: Sr-84 (0.56%), Sr-86 (9.86%), Sr-87 (7.00%) and Sr-88 (82.58%). What is Strontium's atomic mass?
To solve:
(84 X 0.0056) + (86 X 0.0986) + (87 X 0.0700) + (88 X 82.58) = 87.71 u
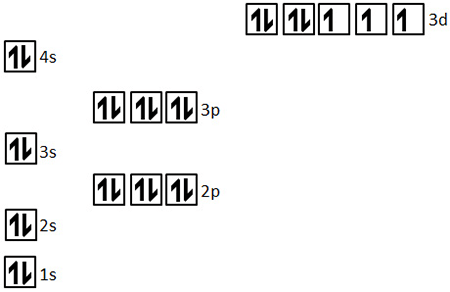
What is the correct orbital notation for Co?
Write the isotope notation for an element that has an atomic number of 16 and a mass number of 33.
The answer should have a S with 33 as a superscript to the left and 16 as a subscript.