This is the electron configuration for carbon.
What is 1s22s222 ?
There are _____ electrons present in this element 1s2 2s2 2p5 3s2 3p6 4s2 3d2 .
What is 21?
Hydrogen has this many valence electrons.
What is 1?
The first energy level can hold this many electrons.
What is 2?
The dots in a Lewis electron-dot structure represent this.
What is the number of valence (outer level) electrons?
This is the lowest part of a wave.
What is trough?
This is the electron configuration for oxygen.
What is 1s2 2s2 2p4 ?
This principle states that single electrons with the same spin must occupy each equal-energy orbital before additional electrons with opposite spins occupy the same orbitals?
What is Hund's rule?
What is 6?
The second energy level can hold this many electrons.
What is 8?
When comparing red and violet light, this one has the greater frequency.
What is violet?
1s2 2s2 2p5 is the electron configuration for this element.
What is fluorine?
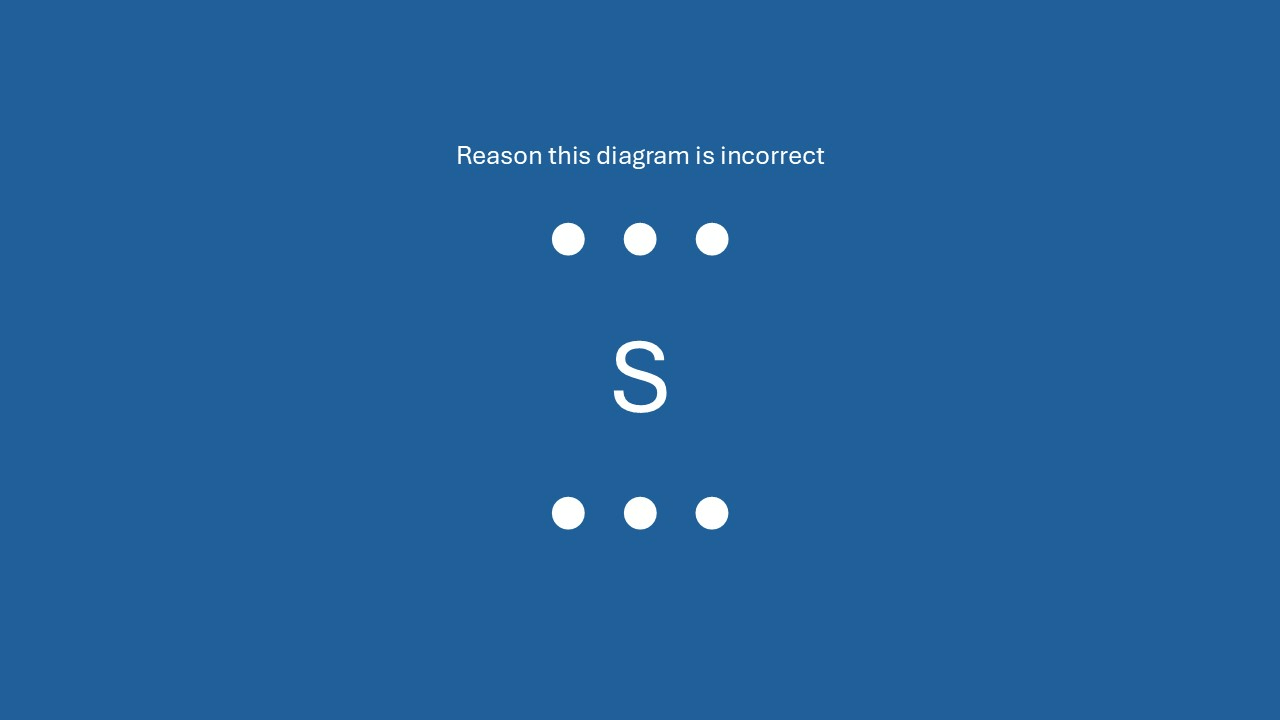
What is only 2 dots can go on each of the four sides?
Barium has this many valence electrons.
What is 2?
These 4 letters represent the sublevels.
What is s, p, d, f?
The _______________ the wavelength, the _____________ the frequency.
Either answer is correct
What is shorter, faster?
What is longer, slower?
The unit used to express energy is the ___________.
What is the joule?
1s2 2s2 2p6 3s2 3p6 4s2 is the electron configuration for this element.
What is Calcium?
The abbreviated electron configuration for aluminum is __________________,
What is [Ne] 3s2 3p1 ?
Iodine has this many valence electrons.
What is 7?
This is the number of electrons held in the d-sublevel
What is 10?
The correct electron dot diagram for helium.
What is He with 2 dots?
This identifies the height or depth of a wave from the baseline.
What is amplitude?
This is the electron configuration for Fe.
What is 1s2 2s2 2p6 3s2 3p6 4s2 3d6?
The period number is equal to the ___________ level.
What is energy?
True or False. All noble gases have 8 valence electrons.
False.
These four letters represent the sublevels (subshells).
What is s, p, d, f.
This is the shape of the s-orbital.
What is spherical?
Wavelength is measured from _________ to _________ or _________ to _________.
What is crest to crest or trough to trough?