This kind of reaction has multiple steps
What is a stepwise reaction?
A reaction in which no mass is added, but mass is taken away
What is an elimination reaction?
This kind of atom is neutral overall, but very reactive
What is a radical?
BDE is an abbreviation of this
What is bond dissociation energy?
The energy required for a reaction to proceed
What is activation energy?
This graph shows a reaction having this kind of mechanism

what is concerted?
A reaction that is a net neutral in terms of mass loss or gain
What is a substitution reaction?
This kind of bond breaking results in radicals
what is homolytic?
BDE is the energy required to break a bond in this way
What is homolytically?
This letter represents the transition state

What is letter C?
This graph shows a reaction with energy
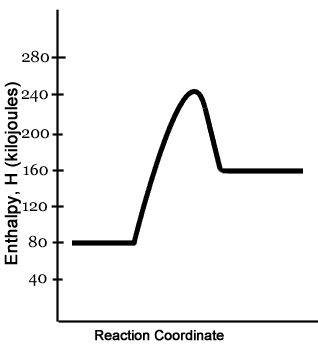
What is Endothermic?
This reaction type typically breaks double bonds
What is an addition reaction
What is Exothermic?
This kind of reaction requires energy input
What is endothermic?
This is how a catalyst decreases the activation energy of a reaction
What is by stabilizing the transition state?
This is the rate-limiting step in this reaction
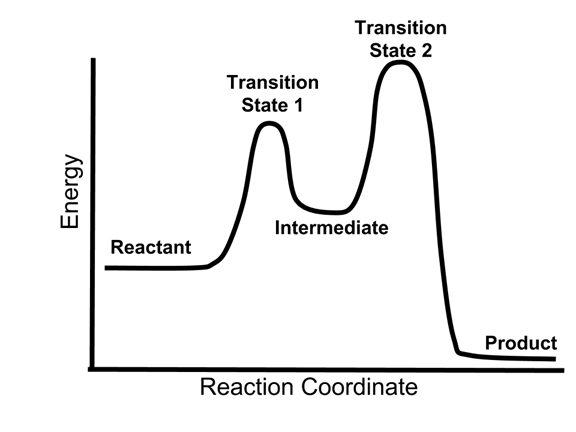
What is step 2
This kind of reaction involves proton transfers.
What is an acid-base reaction?
These kinds of products are favored over the reactants
What are lower energy products?
BDE is used to calculate this
What is Delta H or Enthalpy?
This is the value of Keq when delta G is 0
What is 1?
This graph shows a reaction with a Keq of this size

What is a Keq>1?
Dehydration reactions fall under this category
What is elimination reactions?
This is an unstable positively charged carbon
What is a carbocation?
For the reaction
H3C-Cl + -OH ---> H3C-OH + -Cl
351kJ/mol 389kJ/mol
This is the value of delta H
What is -38kJ/mol?
Bond broken - bond formed
A double-headed arrow indicates this.
What is the movement of two electrons?