The basic unit that composes all matter
What is an atom?
Draw the format for a chemical equation
What is:
Reactants --> Products
Water is this kind of molecule
What is polar?
This atom is the base for all organic compounds
Reactants that bind to enzymes are called this
What are substrates?
A type of bond that occurs when atoms gain or lose electrons
What is an ionic bond?
A factor that affects enzyme activity
What are pH level, temperature, and presence of other substances?
The primary type of intermolecular force
What are van der Waals Forces?
A macromolecule that has a ratio of 1:2:1 (C,H,O)
What is a carbohydrate?
Weak electrostatic attractions between molecules are
What is intermolecular forces?
The octet rule states that atoms seek to have 8 valence electrons in their outermost shell to achieve stability. These two atoms are the exception to the rule
What are hydrogen (H) and helium (He)?
Enzymes often in end with
What is "ase"?
A homogenous mixture is also known as
What is a solution?
Long term energy storage
What is a lipid?
Polymer
What is multiple monomers combined together?
This is created when an atom has the same number of protons and electrons but the number of neutrons changes
What is an isotope?
Balance the equation:
___ Na + ___ NaNO3 --> ___ Na2O + ___ N2
What is:
10 Na + 2 NaNO3 --> 6Na2O + 1N2
(OH)- ions being released gives you this
What is a base?
What is 20?
A combination of two or more substances that retain their individual properties
What is a mixture?
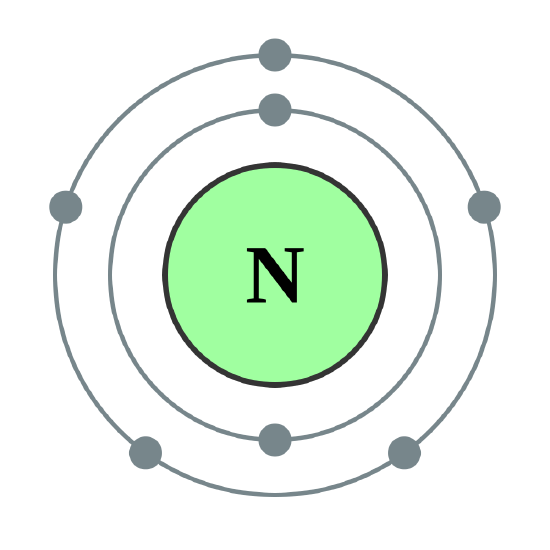
Draw the Bohr model for nitrogen. The atomic number is 7.
Inhibitors are substances that bind to the active site not allowing substrates to bind which slows the reaction rate down. This is an example of one kind of inhibitor
What are competitive, non-competitive, or uncompetitive?
Hydrogen bonds only form when a hydrogen is bonded to one of these elements
What are fluorine, nitrogen, or oxygen?
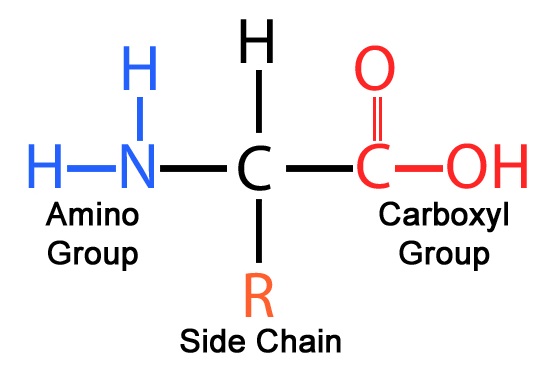
Draw the monomers for the four macromolecule

Forms when two or more different elements combine in fixed ratios thorough chemical bonds
What is a compound?