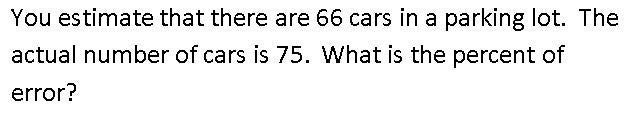What is the name for the law that shows the relationship between volume and temperature?
Charles' Law
True or False: Heating a balloon will cause it to deflate.
false
Particles in motion require ___ energy, which is measured in temperature.
kinetic
When exothermic reactions release heat to the surroundings, it makes the surroundings feel?
hotter
Charles' law about? ....
A) Volume and pressure B) Pressure and temperature C) Mass and volume D) temperature and volume
D
Charles' Law must be used with the ____ temperature scale.
What is Kelvin?
5 L of a gas have a temperature of 250 K. If the new temperature is 300 Kelvin, what is its volume?
6 L
Heat of Vaporization describes the amount of energy needed to change a substance from a liquid to a gas.
True or False?
True
Charles' Law states that volume and temperature are _______ related.
directly?
7 liters of a gas are at a temperature of 300 K. If the volume increases to 7.5 liters, what is the new temperature of the gas?
321.43 K
240 in3 of a gas is at a temperature of 38 K. After an experiment the temperature was 239 in3 at 49 K.
True or False?
False
Charles' Law is about what state of matter?
gas
Runners and athletes may find it harder to perform in cold weather because ____ capacity is reduced. This is an example of Charles' Law.
lung
3.1 m3 of a gas have a temperature of 15°C. What temperature is required to increase the volume to 3.5 m3?
325.33 L
12%
Which of the following did Charles invent? A) Water tanks B) Hydrogen balloons C) gravity
B)
When the volume increases, the temperature...?
increases
A 600 mL sample of nitrogen is heated from 27 °C to 77 °C. What is the final volume?
700 mL
Calculate the energy required to vaporize 3.50 g of water. (40.6 J/g=heat of vaporization)
142.1 J/g