The molecule SO3 has how many valence electrons ?
24
Assign oxidation numbers
Fe2O3
Fe: +3
O: -2
what reaction is this ?
AB + CD → AD + BC ?
Double Replacement
What equation do you use ?
If the volume of a gas at a constant temperature decreases from 4.0 L to 2.0 L, what will happen to its pressure if the amount of gas remains constant?
Boyle's
draw this shape SO3
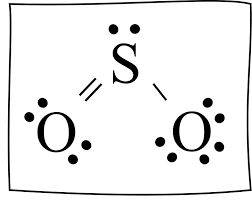
What is molecular geometry ?

Trigonal Pyramidal
What is being reduced, and what is being oxidized?
Cu + 2AgNO3 → Cu(NO3)2 + 2Ag
Reduced: 2Ag
Oxidized: Cu
If you have 1.3 moles of O2 how many particles do you have ?
You have approximately 7.83 x 10^23 particles in 1.3 moles of O2.
What equation are you using here ?
If a gas in a container has a pressure of 2.0 atm at 25°C, what will its pressure be when heated to 50°C if the volume is kept constant?
Gay-Lussac's Law
How many grams of NO are required to produce 145 g of N2 in the following reaction? (MM NO = 30.01 g/mol, MM N2 = 28.02 g/mol)
NH3+ NO → N2+ H2O
On board
Molecular Geometry ?
Linear
Assign oxidation numbers
Na2O2
Na: -1
O: -1
How does one convert from grams --> Mol
Using molar mass
What gas law do you use ?
Calculate the number of moles of a gas that occupies 5.0 L at 3.0 atm and 298 K
Ideal Gas Law
Balance the equation
C4H10 + O2 → CO2 + H2O
2 C4H10 + 13 O2 → 8 CO2 + 10 H2O
Does this need dashes and Wedges ?
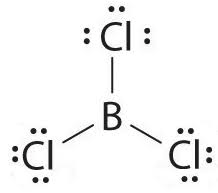
No it is trigonal planar electron group. Only Tetrahedral e group need dashes and wedges
what is being reduced what is being oxidized
Zinc and Copper Sulfate
Balanced Equation: Zn + CuSO4 → ZnSO4 + Cu
Oxidation: Zn → Zn2+ + 2e−
Reduction: Cu2+ + 2e− → Cu
What reaction is this ?
A + O2 = CO2 + H2O
Combustion
Solve
In a mixture of gases, the partial pressure of oxygen (O2) is 0.30 atm, and the partial pressure of nitrogen (N2) is 0.70 atm. What is the total pressure of the gas mixture?
1.0 atm
For the reaction below calculate the grams of H2O produced when 5.0g of O2 react.
2H2 + O2 → 2H2O
What's its molecular geometry ?

Bent, 3 electron groups
2What would the mol ratio step look like if your converting mol O2 to mol H2O in this balanced chemical equation
2H2 + O2 → 2H2O
1 mol O2 / 1 x 2 mol H2O/ 1 mol O2
Arrange the formula to solve for what is missing
A gas sample has an initial pressure of 1.0 atm, a volume of 2.0 L, and a temperature of 300 K. If its volume is changed to 3.0 L and the temperature is changed to 350 K, what will be the final pressure?
P2= P1 xx V1 xx T2 // T1 xx V2
Solve
A gas sample has an initial pressure of 1.0 atm, a volume of 2.0 L, and a temperature of 300 K. If its volume is changed to 3.0 L and the temperature is changed to 350 K, what will be the final pressure?
P2≈2.33atm