Why did models of the atom change over time?
as new technology develops, more detailed views provide scientists with better evidence for what the atom looks like
Of this list, which items represent a CHEMICAL property?
Density, Flammability, boiling point, acidity (pH), malleability, reactivity with water, mass, combustibility
flammability, acidity, reactivity with water, combustibility
Elements in the same group have similar ___
chemical/reactive behaviors
Potential or Kinetic?
Which category would the following fall into:
1) chemical, elastic, and gravitational?
2) radiant, sound, thermal, electrical
1) potential
2) kinetic
What are 5 indications that a chemical change has occurred?
color change
production of a gas
an odor change
heat absorbed/given off
production of light/sound
precipitate is formed
What are the 5 parts of an atom AND what is their charge/location?
Nucleus (center of atom with neutrons and protons)
Electron clouds (outside of nucleus with electrons)
Proton (positive, in nucleus)
Neutron (neutral, in nucleus)
Electron (negative, in electron cloud)
What makes up the bonds that hold molecules and compounds together?
electromagnetic forces between the atoms' opposite charges (protons of one atom and electrons of the other atom)
 What is the:
What is the:
name of this element?
atomic number of this element?
atomic mass of this element?
number of protons of this element?
number of neutrons of this element?
carbon
6
12
6
6
Describe the energy transformation in the following: (3 steps)
turning a flashlight on to see in the dark

1) chemical energy from the battery changes into 2) electrical energy that gets transformed into 3) radiant energy
Why do noble gases rarely form compounds with other elements?
noble gases are chemically stable because they have full valence electrons clouds/energy levels
What is the difference between groups and periods on the periodic table?
Groups are the vertical columns and members have similar reactivity/chemical properties with the same number of valence electrons
Periods are the horizontal rows and members have the same number of electron clouds
What is a chemical reaction?
On the periodic table, where can you find these:
metals?
nonmetals?
metalloids?
left of the stairs
right of the stairs
on the stairs
Identify each type of heat transfer:
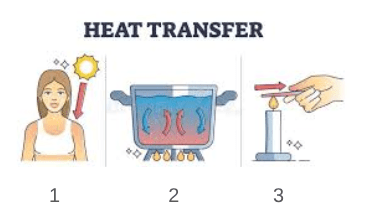
1) radiation
2) convection
3) conduction
What are the negative environmental impacts of using fossil fuels? (give 3)
1) release CO2 in the air, increasing greenhouse gasses and global warming
2) air pollution that leads to respiratory issues
3) habitat destruction/disturbances through mining and drilling
4) takes millions of years to replace them so they can't be replaced as fast as they are used
Where are the most chemically reactive elements on periodic table found?
In groups 1 and 17
What is the difference between IONIC and COVALENT bonds?
ionic bonds form between a metal and a nonmetal when electrons are transferred
covalent bonds form between 2 or more nonmetals when electrons are shared
Metals: shiny (lustrous), malleable, ductile, solids, conducts heat and electricity, high melting point
Nonmetals: dull, solids/liquids/gases, poor conductors of heat/electricity, low melting point, brittle
What happens to the quantity of one type of energy when it is transformed into another type of energy? For example, when a roller coaster begins to move down the first high hill...
The first form of energy begins to decrease as the second form of energy begins to increase
Ex: PE decreases as KE increases
What do the following scientific laws state:
1) Law of Conservation of Mass
2) Law of Conservation of Energy
1) LoCoM: during a chemical reaction, the mass of the reactants must ALWAYS equal the mass of the products
2) LoCoE: energy is neither created or destroyed, it only changes from one form to another
According to the Kinetic Molecular Theory, which state of matter has the highest kinetic energy and how does this affect its volume?
Gas has the highest kinetic energy because its atoms/molecules are moving the fastest, which increases the distance between them causing the volume to increase
Balance the following equation:
__CH4 + __O2 --> __CO2 + __H2O
Coefficients IN ORDER:
1, 2, 1, 2
On which side of a chemical equation will you find:
1) reactants
2) products
1) left side of the arrow
2) right side of the arrow
Look at the image, then answer the questions:
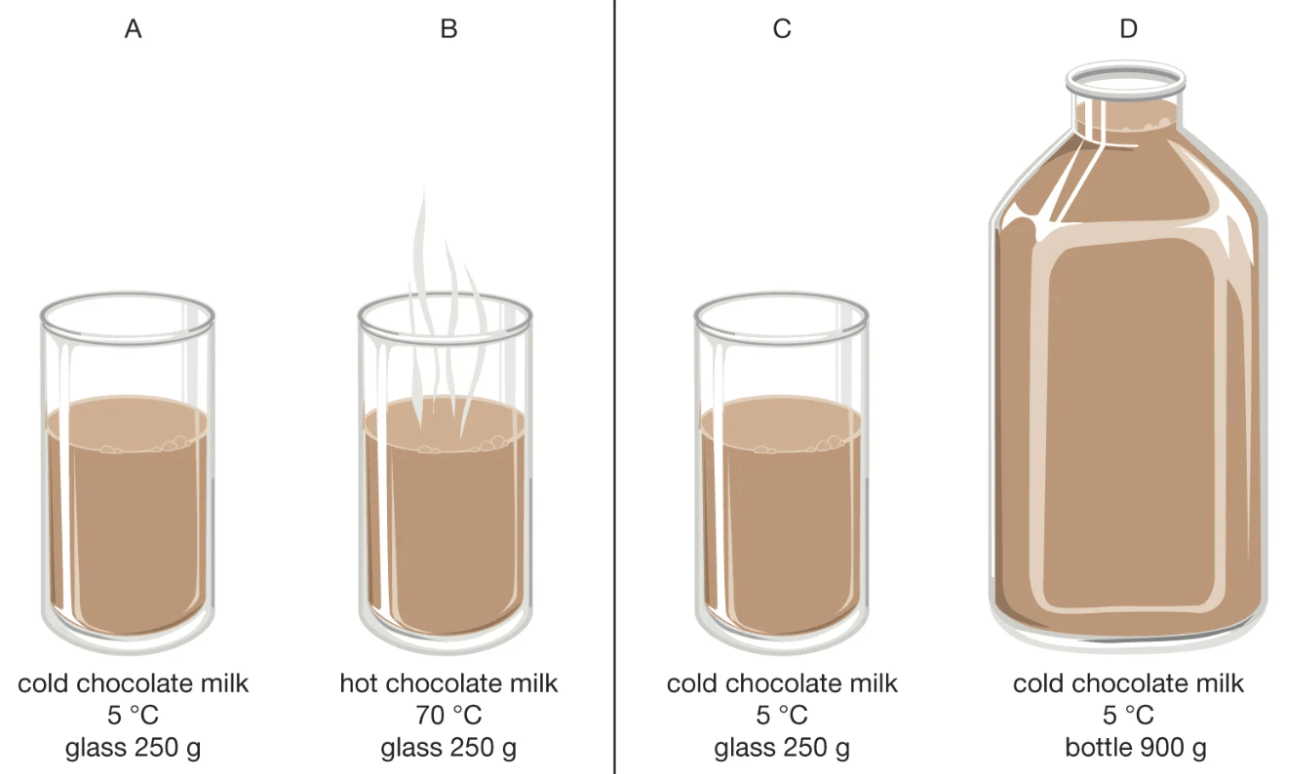
1) Which cup A or B has more thermal energy? How do you know?
2) Which cup C or D will heat up faster? How do you know?
1) B has more thermal energy than A because it is hotter and molecules are moving faster
2) C will heat up faster than D because it has less mass
At which temperature is there the least amount of kinetic energy?
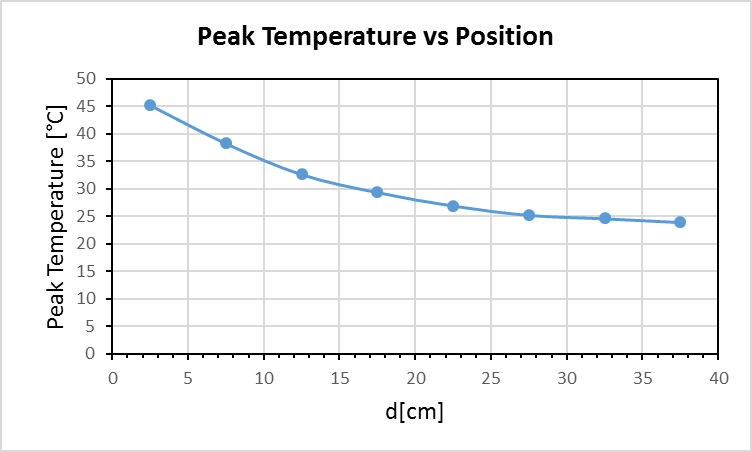
approximately 24oC (less than 25oC)