When you can't see through something it is:
Opaque
Shredding paper is an example of a:
physical change
Is baking a cake a chemical change for the ingredients?
Yes
What type of diagram is this?

Bohr-Rutherford Diagram
Make a word and balanced chemical equation for the following (show states of matter):
Sodium oxide reacts with water to form sodium hydroxide
Sodium Oxide(s) + Water(l) → Sodium Hydroxide(aq)
Na2O(s) + H2O(l) → 2NaOH(aq)
When something reflects light it is:
Lustrous
Baking a cake is an example of a:
Chemical Change
The solid (insoluble particles) that is formed after a chemical reaction is called:
the precipitate
What type of diagram is this?
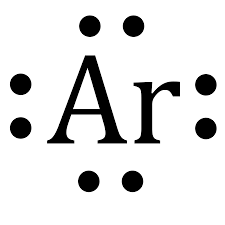
Lewis Dot Diagram
Make a word and balanced chemical equation for the following (show states of matter):
Zinc reacts with hydrogen chloride to form zinc chloride and hydrogen gas.
zinc(s) + hydrochloric acid(aq) → zinc chloride(s) + hydrogen gas(g)
Zn(s) +2HCl(aq) → ZnCl2(s) + H2(g)
The Definition of a Physical Property:
A property that is measurable (quantitative) or something you observe with your 5 senses (qualitative)
Fireworks exploding in the sky are examples of:
Chemical change
If one of the evidences of chemical change occurs does this mean that a chemical change has occurred, 100% of the time?
No, for example gas being produced happens during the boiling of water, but there is no chemical change.
Atoms are made of 3 things, they are:
Protons, Neutrons, and Electrons
Make a balanced chemical equation and a word equation for the following (show states of matter):
When aqueous sodium hydroxide reacts with sulfuric acid, sodium sulfate solution and water are
produced.
2NaOH(aq)+ H2SO4(aq)c Na2SO4(aq) + 2H20(l)
Sodium Hydroxide(aq) + Sulfuric acid (aq) → Sodium Sulfate(aq) + Water(l)
When an object shows the 5 signs of chemical change it has _______ with another object.
reacted
The definition of a physical change:
Physical changes are changes affecting the form of a chemical substance, but not its chemical composition.
What is an evidence of chemical change that is present in all chemical changes?
hard or impossible to reverse
The second last family of the periodic table is:
Halogens
Make a balanced chemical equation and a word equation for the following (show states of matter):
Nitrogen dioxide gas reacts with oxygen gas to produce dinitrogen pentoxide gas.
Nitrogen Dioxide(g)+Oxygen(g) → Dinitrogen Pentoxide(g)
4NO2(g)+O2(g) → 2N2O5(g)
The Definition of a Chemical Property:
Any quality that can be established only by changing a substance's chemical identity. (Several Definitions)
The Definition of a Chemical Change:
A chemical change occurs when a new substance is formed through a chemical reaction
Is mixing vinegar and baking soda going to result in a chemical change?
If it is, what will the products be?
Yes, it will result in a chemical change.
The products will be Carbon Dioxide, Water and Sodium Acetate
The second family in the periodic table is:
Alkaline Earth Metals
Make a balanced chemical equation and a word equation for the following (show states of matter):
Aqueous aluminum nitrate reacts with aqueous magnesium sulfide to produce aqueous magnesium nitrate and solid aluminum sulfide.
2Al(NO3)3(aq)+3MgS(aq)→ 3Mg(NO3)2(aq)+Al2S3(s)
Aluminum Nitrate(aq) + Magnesium Sulfide(aq)→ Magnesium Nitrate(aq) + Aluminum Sulfide(s)