A homogeneous mixture can also go by this term.
What is a solution?
This is an energy of motion
What is kinetic energy?
The total pressure of a mixture of gases is found by doing this?
What is addition?
A solution is unsaturated if the amount of solute lies here 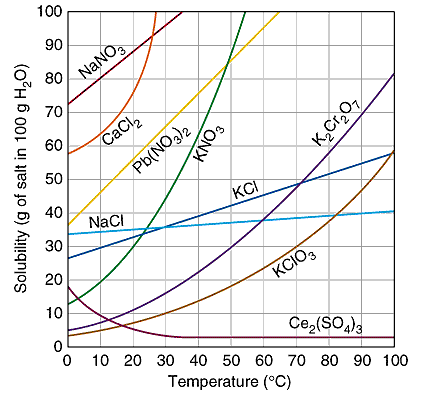
What is below the line (or curve)?
This state of matter is the most compressible
What is a gas?
A substance that gets dissolved
What is a solute?
This is an energy due to relative position
What is potential energy?
This is the SI unit of pressure
What is a pascal?
Starting at this temperature, the solubility of cerium sulfate remains constant. 
What is 40°C?
In this state of matter, the particles have a definite volume and shape
What is a solid?
Water's ability to make so many solutions make it 'universal'
What is a solvent?
This is proportional to the average kinetic energy of molecules.
What is temperature?
A tire on a car should be filled to 32 psi, or this many atmospheres.
What is 2.2 atm?
This curve likely represents a gas..png)
An unstable solution which can spontaneously solidify
What is a supersaturated solution?
At the molecular level, this is a requirement if two things will dissolve in one another.
What is similar polarity?
OR
What is hydration energy > lattice energy?
The amount of heat required to raise the temperature of 1g of substance by 1°C
What is specific heat capacity?
DOUBLE JEOPARDY!!
1 mole of gas at STP would occupy this much volume
What is 22.4 L?
This gas law equation could be modeled with the following curve.
What is Boyle's Law (or P1V1 = P2V2)?
DOUBLE JEOPARDY!!!!
At this temperature, in Fahrenheit units, molecular motion will stop
What is -460°F?
The maximum solubility NaCl of 25°C is 36 g/100 g of water. At the same conditions, 80g of NaCl in 250 g of water, would still allow this much additional NaCl to be dissolved.
What is 10 g NaCl?
These are three things that can be done to increase the rate of a chemical reaction.
What is
increase temperature
increase pressure
increase reactant concentration
add a catalyst
At any given temperature, this noble gas would have the slowest motion out of all the noble gases.
What is Xe?
In the heating curve for water, this is region shows where liquid water exists without changing phases
What is region III?
In order to change liquid water to steam, approximately this much heat is required.

What is 40 kJ?