Energy of motion
What is kinetic energy?
The total pressure of a mixture of gases is found by doing this.
What is addition?
The
DeltaE_{rxn}
for this reaction.
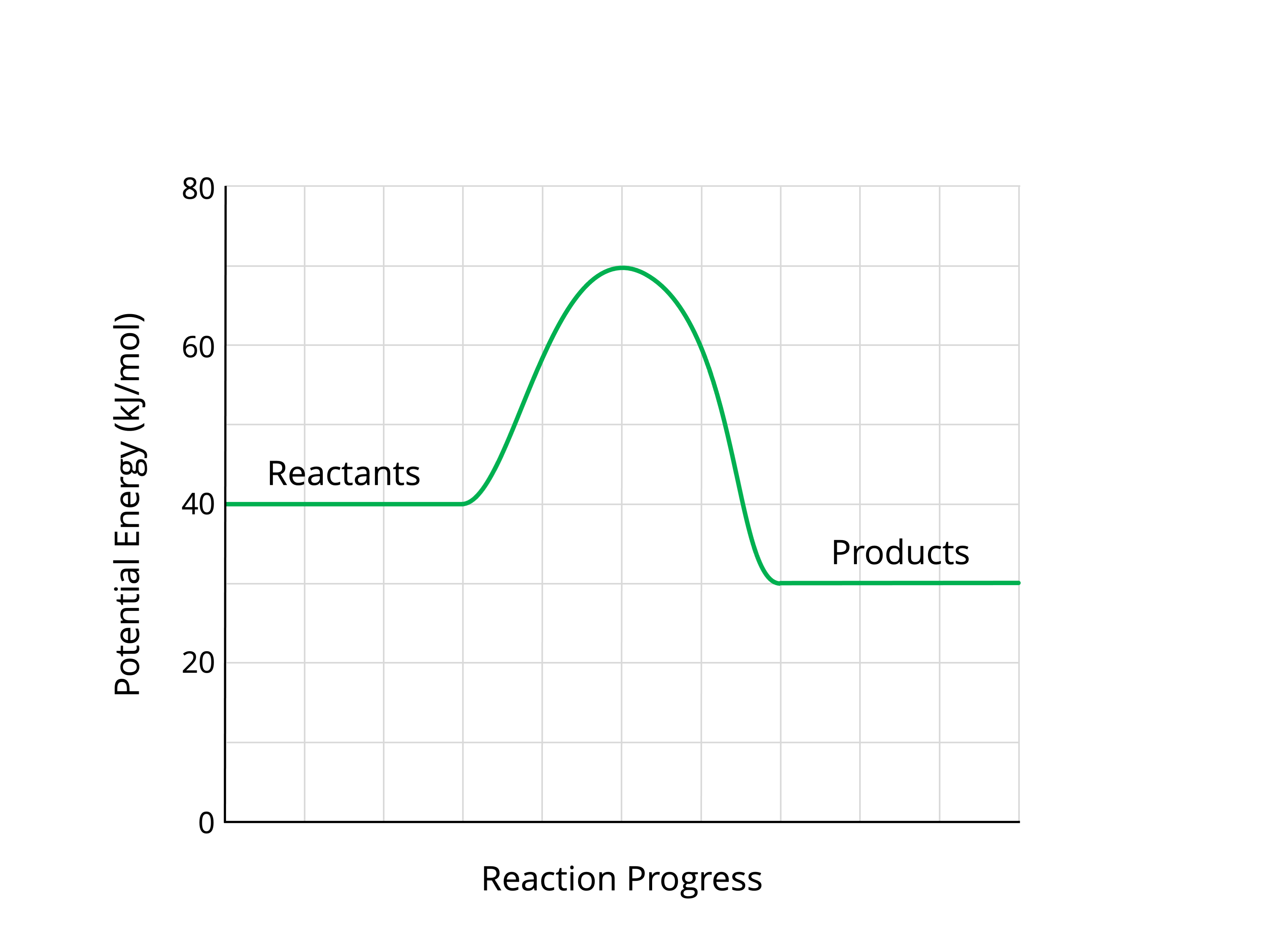
What is -10 kJ?
This state of matter is the most compressible
What is a gas?
The part of the universe we single out to study in a thermodynamic problem.
What is the system?
The oxidation number of all elements.
What is zero.
Most of the energy on Earth comes from here.
What is the Sun?
This is the SI unit of pressure
What is a pascal?
A plot of V vs n, with P and T held constant would look this.
What is a linear (or directly varying) graph?
In this state of matter, the particles have a definite volume and shape
What is a solid?
DOUBLE JEOPARDY!
The type of intermolecular interaction where ionic solutes dissolve in polar solvents.
What are ion-dipole forces?
The substance that gets reduced in a chemical reaction.
What is an oxidizing agent?
This is proportional to the average kinetic energy of molecules.
What is temperature?
A tire on a car should be filled to 32 psi, or this many atmospheres.
What is 2.2 atm?
The hump of this energy reaction curve is called this.

What is activation energy?
A process where a system starts at a low energy state and ends in a higher energy state.
What is endothermic?
A biological substance that speeds up a reaction, but does not get consumed in the reaction.
What is an enzyme?
2H_2+O_2->2H_2O
When 2 moles of hydrogen gas reacts with excess oxygen gas, this is how many moles of H2O would form.
What is 2 moles?
The energy required to complete a phase change.
What is latent heat?
DOUBLE JEOPARDY!!
1 mole of gas at STP would occupy this much volume
What is 22.4 L?
This gas law equation could be modeled with the following curve.
What is Boyle's Law (or P1V1 = P2V2)?
DOUBLE JEOPARDY!!!!
Rounded to the nearest degree, Absolute Zero in units of Fahrenheit.
What is -460°F?
A solution that contains less than the maximum solute that can dissolve is called
What is unsaturated?
What is a double displacement reaction?
These are three things that can generally be done to increase the rate of a chemical reaction.
What is
increase temperature
increase pressure
increase reactant concentration
add a catalyst
The process by which gas escapes from a pinhole.
What is effusion?
In the heating curve for water, this region shows where liquid water exists without changing phases.
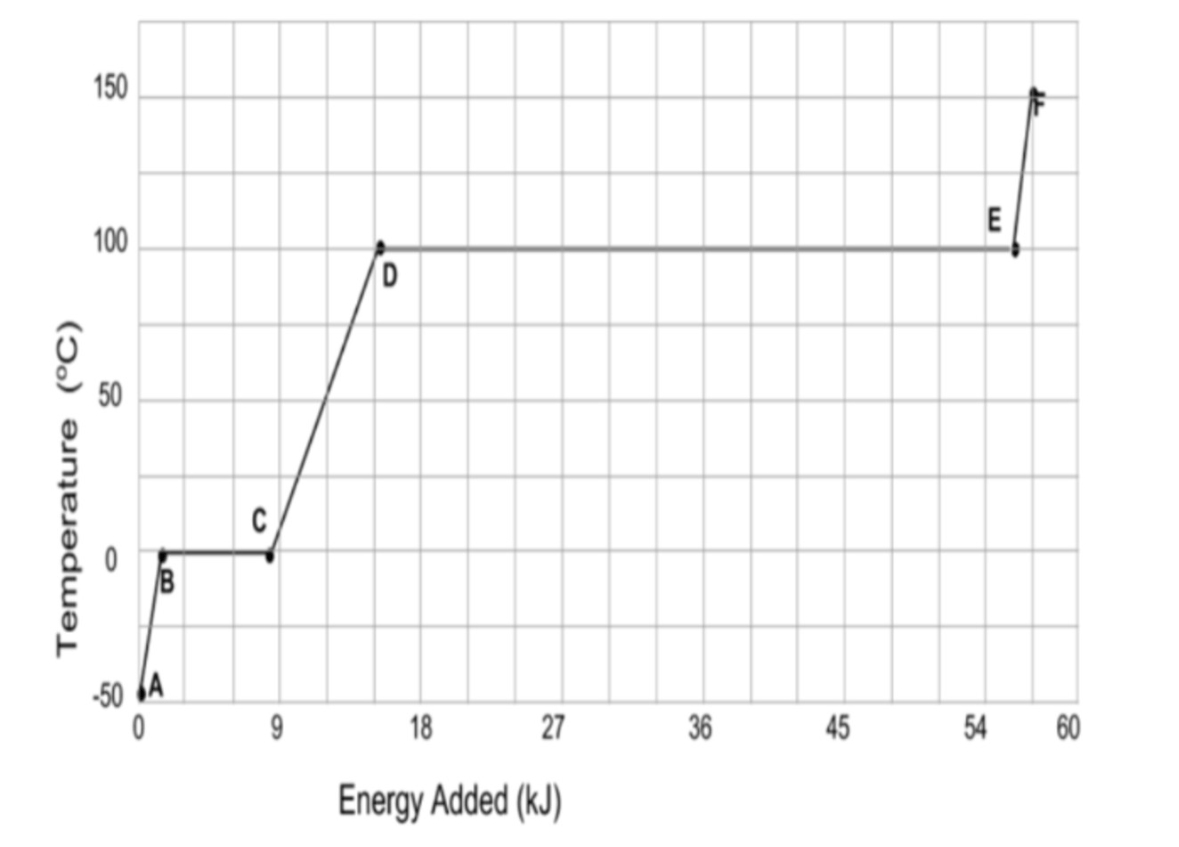
What is region DE?
To change liquid water to steam, approximately this much heat is required.
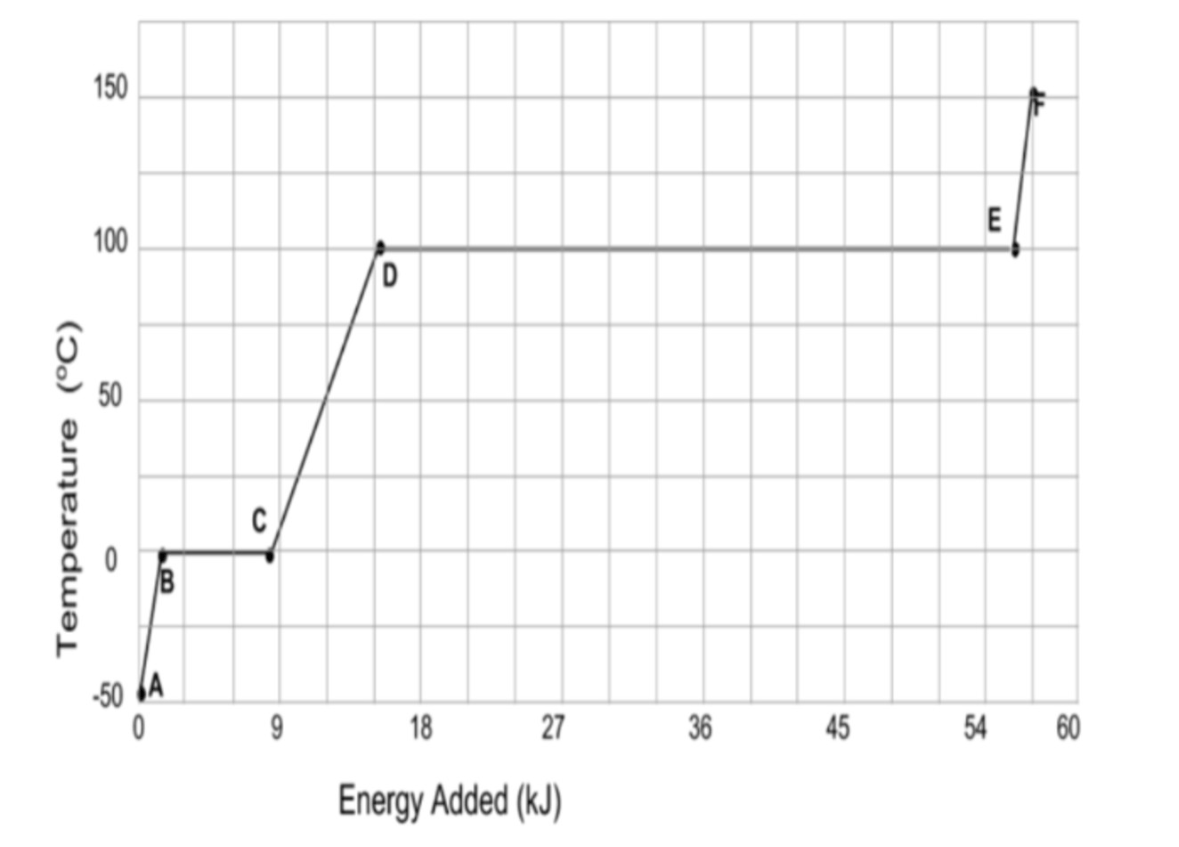
What is 40 kJ?
The resulting molarity of dissolving 1 mole of sugar in enough water to give 100. mL of solution
What is 10 M?
The number of grams of hydrogen that would result from the decomposition of 1 mole of HI.
2HI->H_2+I_2
What is 1 gram?
DOUBLE JEOPARDY
The amount of calories needed to raise the temperature of 1 g of water by 1 kelvin.
What is 1 calorie?
DOUBLE JEOPARDY!
The framework that explains the gas laws
What is kinetic molecular theory?
What is the pressure increase by 2x?
The name of the phase change when a gas becomes a solid.
What is deposition?
The capacity to do work.
What is energy?
DOUBLE JEOPARDY!
2H_2+O_2->2H_2O
When 4 g of hydrogen gas reacts with 32 g of oxygen gas, this is how many grams of H2O would form.
What is 2 moles?