This is the number of meters in a kilometer.
What is 1000?
One mole of any substance contains this many particles.
What is 6.02x1023 particles?
It produces H+ ions in solution.
What is an acid?
This gives two molecules when it's completely hydrolyzed.
What is a disaccharide?
This law states that the solubility of a gas in a liquid increases as the gas pressure above the liquid increases.
What is Henry's Law?
This is the number of grams in 5.37 lb, rounded to the appropriate number of significant figures and with the correct unit.
What is 2440 g?
This is the number of moles of oxygen needed to react with 6.0 moles of magnesium:
2 Mg + O2 -> 2MgO
What is 3.0 moles?
This is conjugate base of H2CO3.
What is HCO3-?
This is the number of unique groups that must be on a carbon for it to be chiral.
What is 4?
It's the name of the following organic molecule.
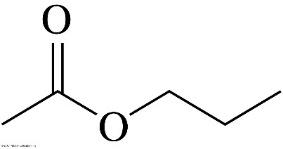
What is propyl ethanoate?
It's the name for CS2.
What is carbon disulfide?
This is 135 F when converted to degrees Celsius.
What is 57.2 C?
This unit is also known as moles per liter.
What is molarity?
This is what cell walls are composed of, according to the fluid-mosaic model of the cell.
What are phospholipid bilayers?
This type of reaction always results in the production of carbon dioxide and water.
What is combustion?
It's the formula for the compound iron(II) hydroxide.
What is Fe(OH)2?
This is the number of grams needed to prepare 150 mL of a 2.50 M HCl solution.
What is 13.7g?
These are the six strong acids.
What are hydroiodic acid, hydrobromic acid, perchloric acid, hydrochloric acid, sulfuric acid, and nitric acid?
These are compounds that have the same molecular formula but different arrangements of atoms.
What are structural isomers?
This ideal gas law relates pressure and volume.
What is Boyle's Law?
Multipart Question!
For the compound CHCl3, determine the number of valence electrons, draw the best Lewis Structure possible, determine the molecular geometry, and determine if it is polar or nonpolar.

26 Valence electrons
tetrahedral molecular geometry
polar
This is the number of grams of CO2 that can be produced when 125g of C2H2 is combusted.
2C2H2 + 5O2 -> 4CO2 + 2H2O
What is 423g?
This is the molarity of 25.0 mL of HCl solution that was titrated with 32.8 mL of 0.428 M NaOH.
What is 0.562 M?
This type of enzyme inhibitor binds to the enzyme away from the active site and can be reversed by reducing inhibitor concentration.
What is a noncompetitive inhibitor?
This is the correct dose, in grams, of an intramuscular medication, prescribed at 3.000 mg/kg of body mass, to a patient that weighs 175 lb.
What is 0.239g?