(Chapters 1-3)
(Chapters 1-3)
(Chapters 4, 6-7)
(Chapters 8-10)
(Chapters 11-12, 14)
This is the number of meters in a kilometer.
What is 1000?
This is the name for a positively charged ion.
What is cation?
This is the relationship between volume and temperature, according to Charles's Law.
What is directly related?
This is what we call the study of carbon compounds in chemistry.
What is organic chemistry?
This is the number of grams in 5.37 pounds, rounded to the appropriate number of significant figures and with the correct unit. (2.20 lb =1 kg)
What is 2440 g?
This is the name for the molecule N2O5.
What is dinitrogen pentoxide?
This is the name for the component in a mixture that you have the least of.
According to IUPAC rules, this is the part that is selected for the main carbon chain when naming an organic molecule.
What is the longest continuous carbon chain?
This is the temperature scale in which "zero" means all motion has stopped.
What is Kelvin?
This is the type of reaction the following is classified as.
C5H8 + 7O2 -> 5CO2 + 4H2O
What is combustion?
This is how you calculate molarity.
What is moles divided by liters?
This is the correct formula for an alkene with 8 carbon atoms.
What is C8H16?
It's the prefix for 10-9 in the metric system.
What is nano?
This is the number of electron groups required to have a trigonal planar electron geometry.
What is 3?
It's the name for the reaction between an acid and a base.
What is neutralization?
Comparing propanal to propanoic acid, this is the reason why propanoic acid has a higher boiling point.
What is hydrogen bonding?
DOUBLE ANSWER!
These are the rules for significant figures for:
- Addition and subtraction
- Multiplication and division
What is the fewest number of decimal places?
What is the fewest number of significant figures?
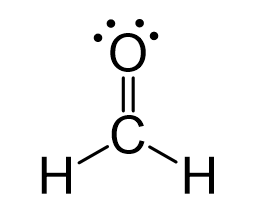
This is the correct Lewis Structure for CH2O.
What is:
This is what happens to pH when respiratory alkalosis is occurring.
What is increase?
It's the name for the following organic molecule:
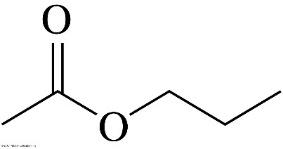
What is propyl ethanoate?