Which one of the following compounds contains both ionic and covalent bonds?
A. LiF D. C2H5OH
B. NaNO3. E. C6H12O6
C. MgCl2
B. NaNO3
Determine the final temperature when a 25.0 g piece of iron at 85.0 °C is placed into 75.0 grams of water at 20.0 °C. *C of Fe = 0.45
22.25 oC
(25.0)(85.0 − x)(0.45) = (75.0)(x − 20.0)(4.184)
13.002x = 289.29
x=22.25 oC
DRAW IT OUT
What is the Lewis Structure for CH2O

Absorbance measurements taken as a function of concentration for a series of known analyte concentrations yielded a calibration line of y = 20215x + 0.0064. What concentration of the analyte would be expected to yield an absorbance of 0.5780? Enter your answer in scientific notation using 3 significant figures.
2.83 x 10-05
0.5780 = 20215x + 0.0064
x = 2.83 x 10-05
Fill in the blank:
1. Methane
2. _______ (25 points)
3. _______ (25 points)
4. _______ (25 points)
5. _______ (25 points)
6. Hexane
2. Ethane
3. Propane
4. Butane
5. Pentane
What element is the most metallic?
A. Ca D. Cs
B. Rb. E. Al
C. Ga
D. Cs
After a run in the morning, a student is resting during her CHM 11500 lecture. Her resting pulse rate is 77 beats per minute and her heart pumps about 75 mL of blood per beat. Circulating all of the blood through the heart takes about 1 min for her at rest.
Approximately how much blood is in her body?
Provide your answer in m3 using 2 significant figures.
5.8 x 10-3 m3
77 x 75 = 5775 ml/min
5775ml/min x 1cm3/1ml x 1m3/1003cm3 = 5.8x10-3
How many shared electrons does the nitrogen in the cyanate ion NCO– have in the most preferred Lewis structure?
Enter a whole number. Do not use decimals, fractions, or scientific notation.
6

Calculate the enthalpy for the reaction:
2C (s) + H2 (g) → C2H2 (g) ΔH° = ??? kJ/mol
Given the following thermochemical equations:
C2H2 (g) + 5/2 O2 (g) → 2CO2 (g) + H2O (ℓ) ΔH° = −1299.5 kJ/mol
C(s) + O2 (g) → CO2 (g) ΔH° = −393.5 kJ/mol
H2 (g) + 1/2 O2 (g) → H2O (ℓ) ΔH° = −285.8 kJ/mol
Enter your answer using four significant figures.
226.7 kJ/mol
What does a solution go through when it transitions from solid -> gas? Gas -> solid?
1. Sublimation
2. Deposition
What is the electron Configuration of Hf+?
[Xe] 6s24f145d
An atom of 81Br has an experimentally determined nuclear mass of 80.9163 amu. Calculate the mass defect, Δ𝑚, in atomic mass units (amu).
Proton: 1.0072765 amu
Neutron: 1.0086649 amu
0.7370 amu
35(1.0072765) + 45(1.0086649) = 81.653263 amu
Δ𝑚=81.653263 − 80.9163 = 0.7370 amu
Identify the functional groups present in the compound:
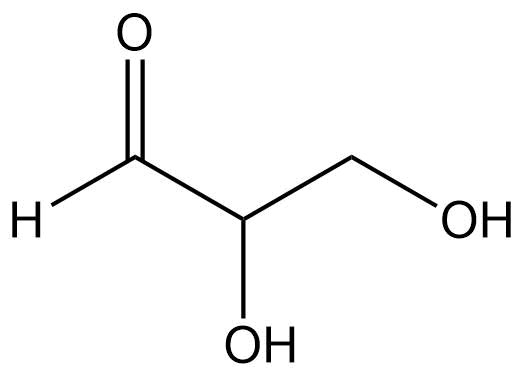
Alcohol
Aldyhide
How many atoms of oxygen are in 2.25 mol of copper(II) phosphate (phosphate is PO43- )? Enter your answer using the appropriate number of significant figures.
1.08 x 1025
2.25 mol Cu3(PO4)2 x 8mol O/1mol Cu3(PO4)2 x (6.022 x 1023)/1mol O = 1.08 x 1025
Rank the 6 Intermolecular Forces from strongest to weakest.
*Core 3 = 50 points*
Ion-Dipole, Hydrogen Bonding, Dipole-Dipole, Ion Induced Dipole, Dipole Induced Dipole, London Dispersion
The three principle quantum numbers n,l,ml determine the:
size, shape, and orientation of an orbital respectively.
SURPRISE X2 POINTS
Determine the molality and mole fraction of concentrated aqueous HCl, which is 12.0 M and has a density of 1.19 g/mL.
molality= 15.946759087 m
Mole Fraction= 0.223041705535
How many sigma and pi bonds are there is serotonin?
Below is the skeletal structure of serotonin:
Sigma: 18 bonds
Pi : 4 bonds
SURPRISE X2
A solution is made by dissolving 0.420 mol of nonelectrolyte solute in 695 g of benzene. Calculate the freezing point,Tf, and boiling point,Tb, of the solution.
Kf = 5.12 Freezing Point: 5.49
Kb = 2.53 Boiling Point: 80.1
Tf = 2.40 ∘C
Tb = 81.63 ∘C
𝑚=0.420mol solute / 0.695kg solvent= 0.604
Δ𝑇f = (5.12 ∘C/𝑚)( 0.604𝑚) = 3.09 ∘C
𝑇f = (5.49−3.09)= 2.40 ∘C
Δ𝑇b=(2.53 ∘C/𝑚)( 0.604𝑚)= 1.53 ∘C
𝑇b = (80.1+1.53) = 81.63 ∘C
Determine the Hybridization (ex. sp), bond angle, and shape of the Carbon atom in the given structure.
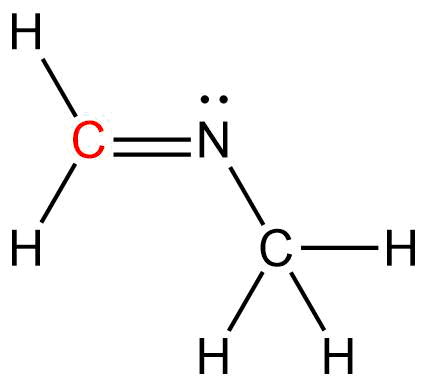
Hybridization: sp2
Bond Angle: ~1200
Shape: Trigonal Planar
POINTS X1 (no half credit)
Calculate the number of orbital sublevels and number of orbital energy levels for Nb
7 sublevels
5 energy levels
Sublevels = 2l+1: l = 3; 2(3)+1 = 7 sublevels
Energy Levels = n: n = 5 energy levels
A moon rock collected by a U.S. Apollo mission is estimated to be 3.70 billion years old by uranium/lead dating. Assuming that the rock did not contain any lead when it was formed, what is the current mass of 206Pb in the rock, if it currently contains 1.190 g of 238U? The half-life of 238U is 4.47×109 years.
0.798
Mf=Mi(1/2)t/t1/2 -> Mi=(Mf)/(1/2)t/t1/2
Mi=2.112 g
2.112-1.190 = 0.922 g
0.922g U x 1mol U/238g U x 1mol Pb/1mol U x 200g Pb/1mol Pb = 0.798g
DRAW IT OUT
Which is the most favorable resonance structure of NCO-?

Calculate the longest wavelength of light, in nm, capable of dissociating the Br-Br bond in one molecule of Br2 if the bond energy, or bond dissociation energy, is 210 kJ/mol. Enter your answer using 3 significant figures.
570 nm
E= (210 x 103)/(6.022 x 1023) = 3.487 x 10-19
Lamda = hc/E
Lamda = ((6.63 x 10-34)(3 x 108))/3.487 x 10-19
Lamda = 5.704 x 10-07 m
5.704 x 10-07 m x 1nm/10-09m = 570 nm
A CHM 11500 student performed the “Which Cereal Has the Most Iron?” lab and found that the concentration of Fe3+ in her original (undiluted) sample solution prepared from her crushed cereal sample was 3.76 x 10-3 M. The volume of her original (undiluted) sample solution solution was 100.0 mL. Calculate the mass of Fe (in mg) in her cereal sample.
Enter your answer using 3 significant figures and standard notation.
21.0 mg
