party!
Chemistry?
- Electron domain
________ is a representation of an atom’s valence electrons by using dots and indicates by the placement of these how the valence electrons are distributed in the molecule
What is Lewis structure?
Determines what resonance structure would be favored
What is the smallest formal charge?
A molecule that has no central atom
What is a binary linear molecule?
If an s orbital and two p orbitals combine, this hybridization results
What is sp2?
The acronym VSEPR stands for this phrase
What is Valence Shell Electron-Pair Repulsion?
The electron dot structure of CCl4
What is 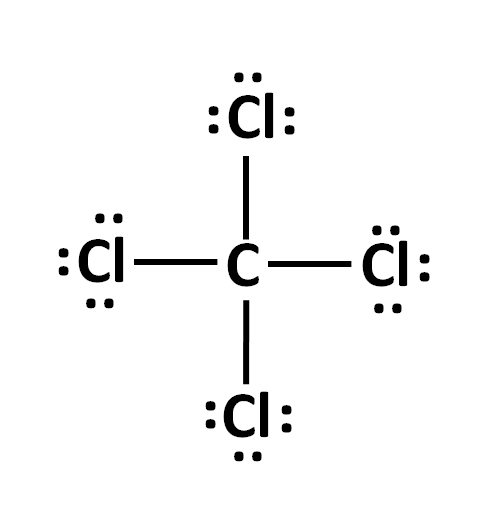
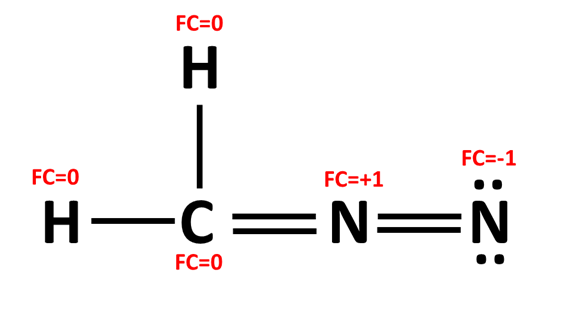
The formal charge of N in the structure below:
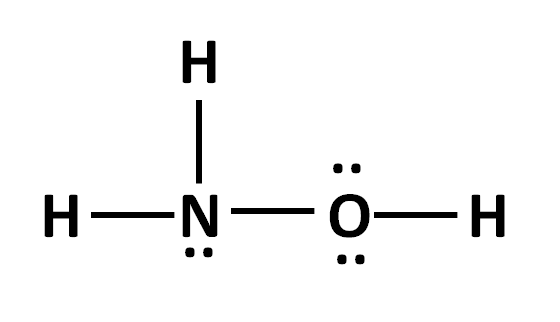
What is zero?
DAILY DOUBLES!
Number of charge clouds around the central atom that has a seesaw geometry
What is five (5)?
Hybridization for atoms that have 2 charge clouds?
What is sp?
According to the VSEPR, electron domains orient themselves to minimize these
What is repulsion?
The electron-dot structure of NO+
What is
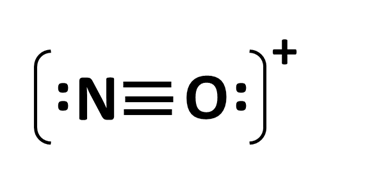
DAILY DOUBLES!
The formal charges to each atoms in ClO2- (see structure below)?
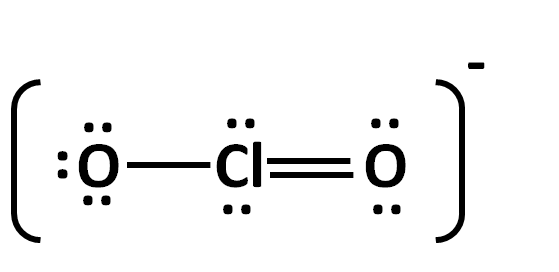
What is -1 for O(left), 0 for Cl and 0 for O(right)?
Shape of a molecule that has a central atom with two lone pairs and four bonds to other atoms
What is square planar?
Number of hybrid orbitals that results in the combination of an s orbital and three p orbitals
What is four (4)?
Type of bond that results in a direct or head-on overlap of orbitals
What is sigma bond?
DAILY DOUBLES!
The third-row element, X, that forms the following ion (XH4)+?
What is Phosphorus?
The three resonance structures for thiocyanate ion, [SCN]- whose connections are shown below:
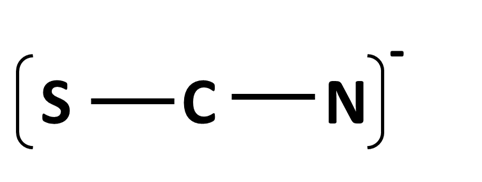
What is
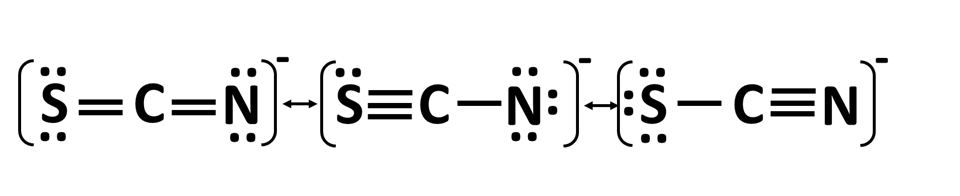
Molecular geometry of ammonia, NH3
What is trigonal pyramidal?
Hybridization around the central atom in CH4
What is sp3?
Total number of sigma and pi bonds in O3
What are 2 sigma and 1 pi bonds?
The electron dot structure for molecule with the following connections, showing lone pairs and any multiple bonds: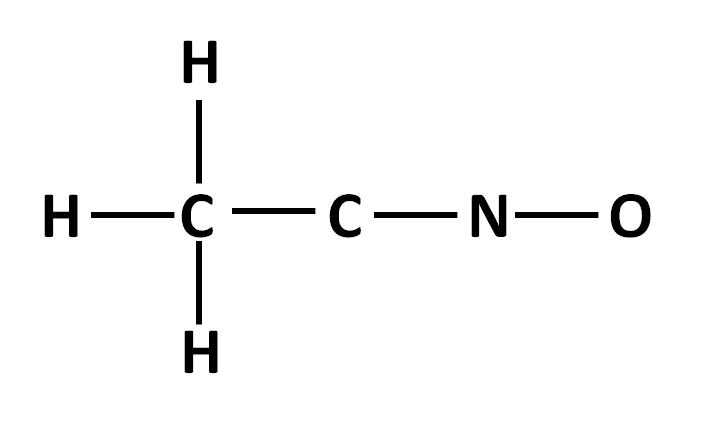
What is
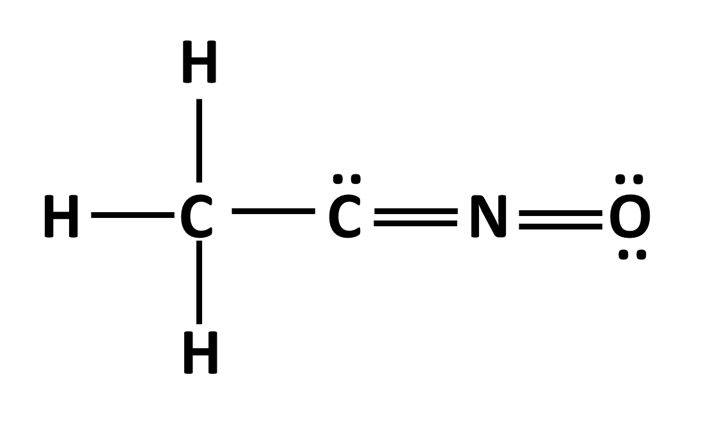
Determine the formal charge of each atom in the structure below.
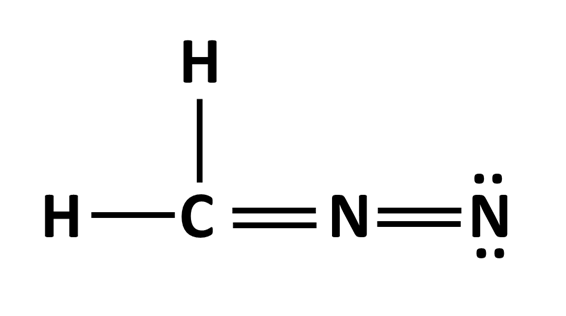
What is
The shape of NO3-
What is trigonal planar?
DAILY DOUBLES!
Hybridization around the central atom in O3
What is sp2?
DAILY DOUBLES!
Total number of pi and sigma bonds in acrylonitrile (see structure):
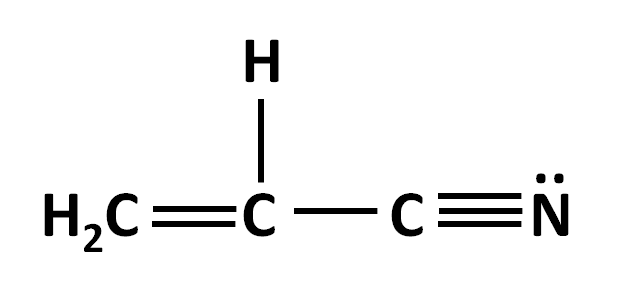
What are 4 sigma and 3 pi bonds?