What is the formula for the Ideal gas law?
What does R equal to?
formula: PV=NRT
R= 0.8206 Lxatm/molxk
A sample of krypton gas occupies 75.0 mL at 0.400 atm. If the temperature remained constant, what volume would the krypton occupy at 4.00 atm?
V= 0.00750 L
Draw the expanded structure for butane.

name this functional group:
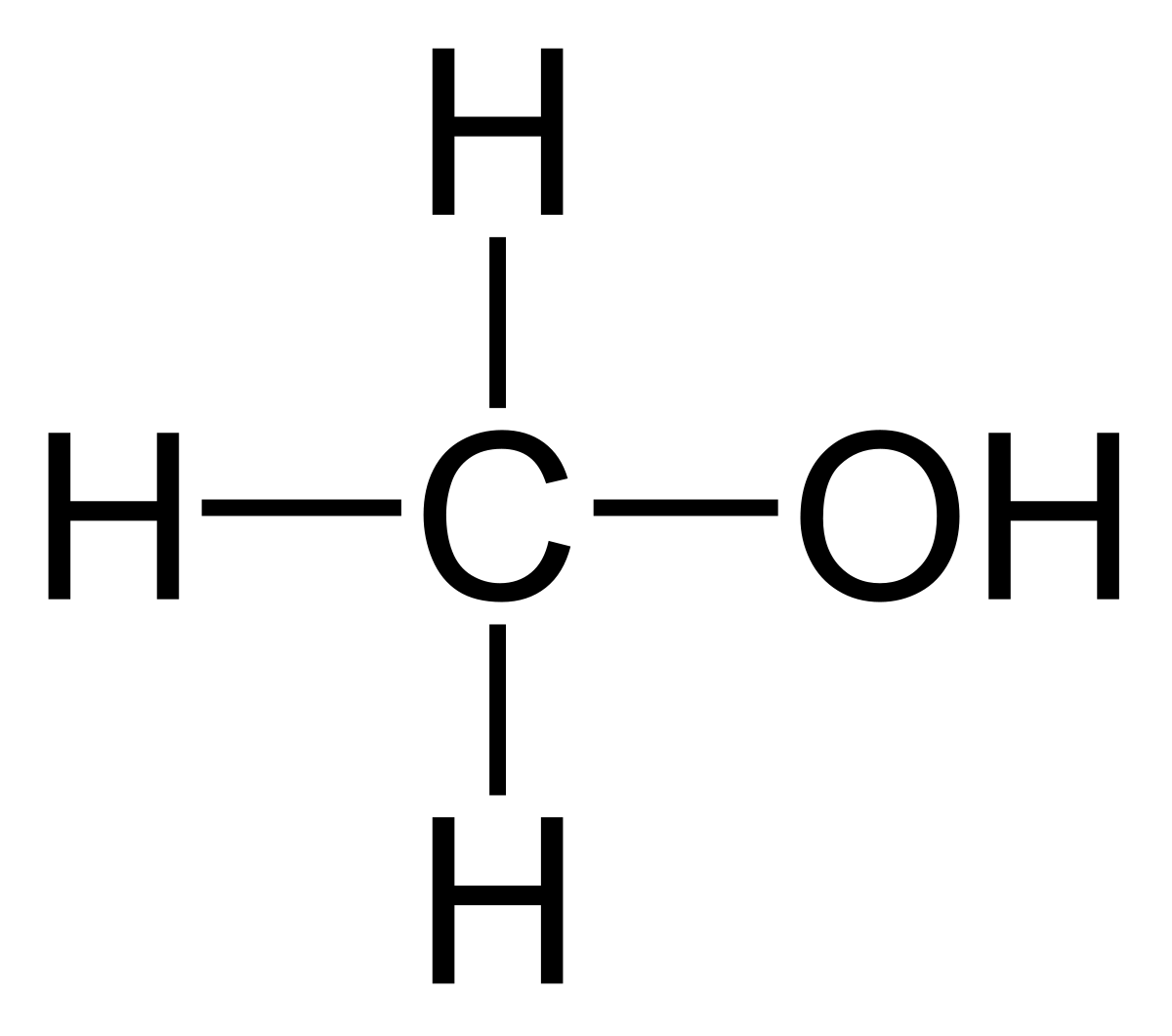
methanol
What is the definition for atmospheric pressure?
The pressure exerted by molecules of air from the atmosphere
What formula will be used if you have the numbers below?
-1 atm
-2 L
-4 atm
-11 L
formula= P1V1=P2V2
(Boyles law)
2.15 moles of oxygen occupies 650. mL. How many moles of oxygen would be present if the volume were 1300. mL, assuming pressure and temperature remain constant?
n2=4.30 moles
Name the structure of this:

butanol
What is the definition of organic chemistry?
The branch of chemistry that focuses on carbon containing compounds and their chemical reactions
What is an example of Charles law?
Tire pressure shrink when cold (winter time) and expands when hot (summer time)
To what temperature must 6.50 moles of a gas have to be heated in a 15.0 L container in order for it to exert a pressure of 7.50 atm?
T=211 K
What's the name of this:
2-octene
How would you describe benzene?
A ring structure with six carbon and six hydrogen and alternating double bonds.
What are the four types of organic chemistry?
1.) alkanes
2.) alkenes
3.) alkynes
4.) aromatic hydrocarbons
Give an example of Boyles law:
exhaling:
-lung volume will decrease and pressure will increase
Inhaling:
-lung volume will increase and will pressure decrease
If I have 45 liters of helium in a balloon at 25°C and increase the temperature of the balloon to 55°C, what will the new volume of the balloon be?
V2= 50. L
What is the name of this:
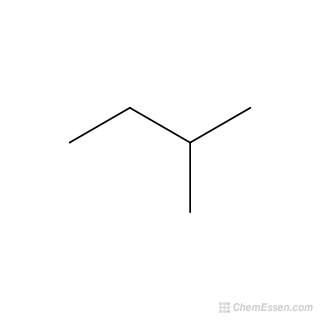
2-methyl butane
What is the name of the alcohol that is found in alcoholic beverages?
Ethanol
What are structural isomers?
The same molecular formula, but different bonding arrangement
What is the kinetic theory of gases?
-gas has small particles
-move randomly
-no attraction towards each other
-small volume
-in constant motion
-The kinetic energy increase with temperature
A 35 L tank of oxygen is at 315 K with an internal pressure of 190 atmospheres. How many moles of gas does the tank contain?
n= 260 moles
What is the name of this:

Where are the hydrogens at on a cis isomer?
On the same side
What is boiling point defined as?
The temperature at which liquid's vapor of pressure is equal to the pressure of the gas above it.