Which element has the larger atomic radius, selenium or oxygen?
What is selenium?
What is the electron pair geometry of CCl4?
What is tetrahedral?
What is the speed of light?
What is 3.0 x 108 m/s?
What type of bonds or bonding is present in Be (s)?
(covalent, metallic, or none)
What is metallic?
How many orbitals are in the d subshell?
What is 5?
What is the molar mass of methane?
What is 16.042 g/mol?
What is the most electronegative element?
What is fluorine?
What is the molecular geometry of H3O+?
What is trigonal pyramidal?
What is Planck's constant?
What is 6.63 x 10-34 J*s?
What type of bonds or bonding is present in F2 (g)?
(covalent, metallic, or none)
What is covalent?
What are the quantum numbers for argon?
What is n=3, l=1, ml=1, ms=-1/2?
What is the molar mass of phosphoric acid?
What is 97.994 g/mol?
Arrange the following elements in order of increasing ionization energy: beryllium, magnesium, and strontium
What is strontium, magnesium, and beryllium?
Is hydrogen cyanide (HCN) polar or non polar?
What is polar?
What is the wavelength of a light with a frequency of 7.26 x 1014 Hz? What region of the electromagnetic spectrum would this lie in?
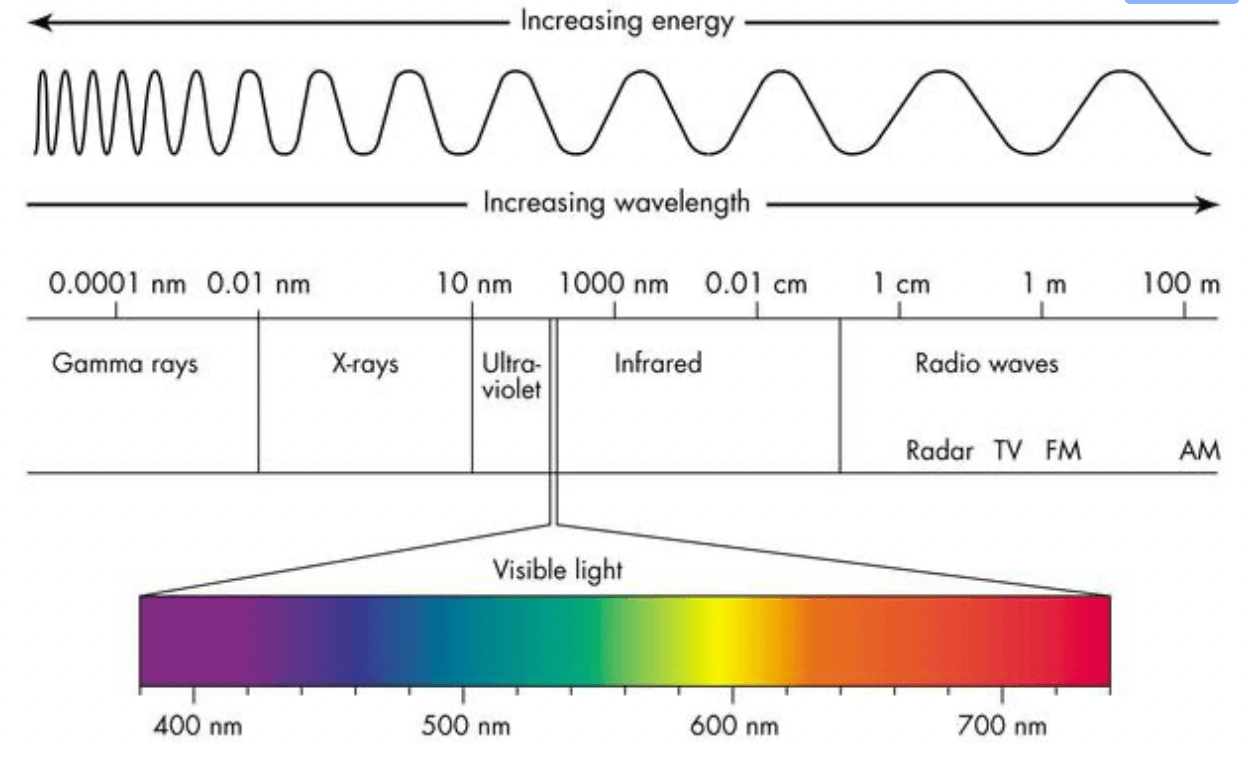
What is 4.13 x 10-7 m (or 413 nm), visible - violet?
DAILY DOUBLE :
draw a duck and explain the interaction between two ducks following a Lenard jones potential graph.
quack
What element are I- and Ba2+ isoelectronic with?
What is xenon?
What is the limiting reagent when 7 mol NaCl reacts with 4 mol Mg(BrO4)2?
What is NaCl?
Which is larger the atom or the ion: Al or Al3+
What is Al?
What is the hybridization of the atom indicated by the arrow?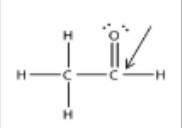
What is sp2?
A hydrogen-hydrogen covalent bond (in H2) requires 7.2 x 10-19 J of energy to break the bond in the molecule. Calculate the wavelength (in nm) of this light. What region of the electromagnetic spectrum does this fall in?
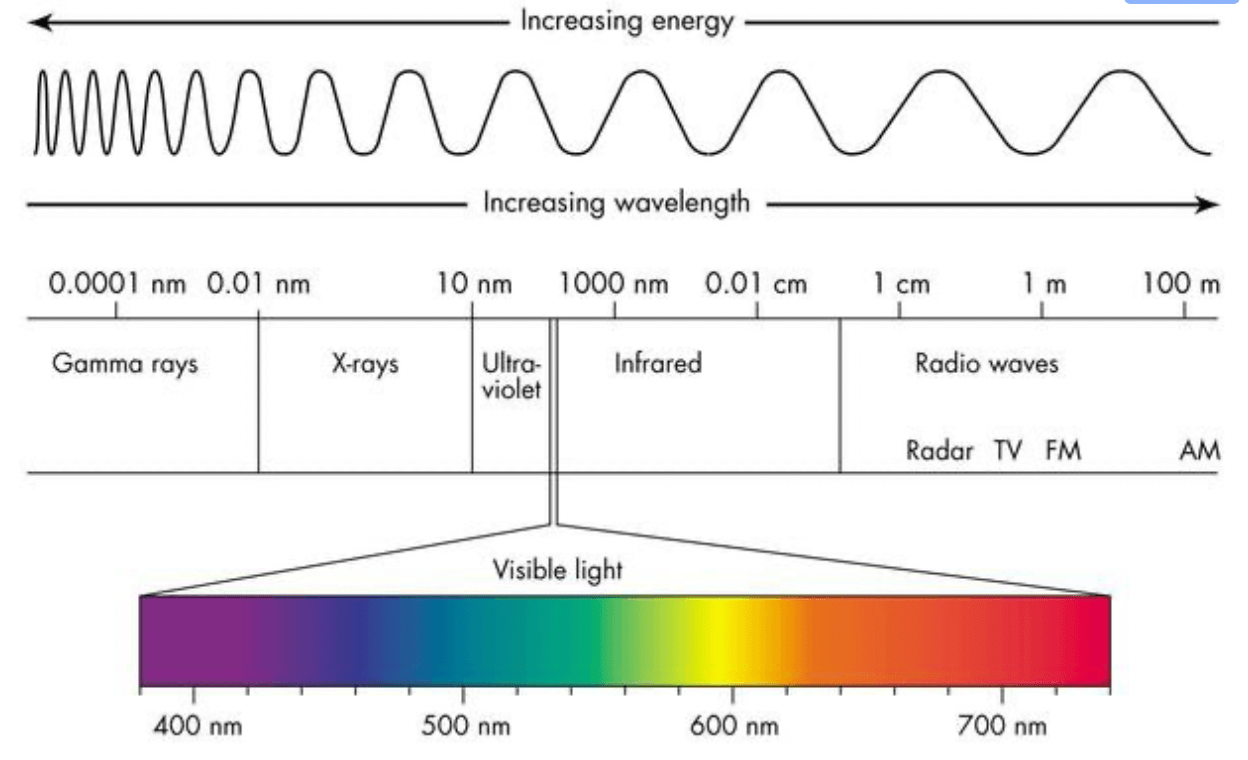
What is 276 nm? What is UV?
Chlorine (Cl2) has a melting point of -101.5oC and a boiling point of -34.04oC. At temperatures below -101.5oC, what types of bonds and/or interactions are present in Cl2?
What is covalent bonds and LDFs?
What are the quantum numbers for iridium (Ir)?
What is n=5, l=2, ml=-1, ms=-1/2?
What mass of carbon dioxide can be produced if 10 g of methane reacts with excess oxygen?
2 C4H10 + 13 O2 --> 8 CO2 + 10 H2O
What is 30 g CO2?
List the following elements in order of decreasing atomic radius: S, Cl, Al, and Na
What is Sodium, Aluminum, Sulfur, Chlorine?
What are the strongest interactions between three HCN molecules? (draw the molecules)
What is dipole-dipole interactions?
The energy to break 1 mole of C-C bonds (that is, 6.022 x 1023 C-C bonds) is 348 kJ/mol. What would be the minimum frequency of a photon required to break a single C-C bond?
What is 8.72 x 1014 Hz?
Chlorine (Cl2) has a melting point of -101.5oC and a boiling point of -34.04oC. At temperatures above -34.04oC, what types of bonds and/or interactions are present in Cl2?
What is covalent bonds?
What is the full electron configuration for ruthenium (Ru)?
What is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d6?