Determine amount (in moles) and the mass (in grams) of copper(II) carbonate needed to produce 1.500 kg of copper(II) oxide. (CO2 is the other product.)
CuCO3 -> CuO + O2
18.39 mol CuCO3, 2.310 x 103 g CuCO3
A 2.50-L volume of hydrogen measured at –196 °C is warmed to 100 °C. Calculate the volume of the gas at the higher temperature, assuming no change in pressure.
12 L
A system releases 511 kJ of heat and does 125 kJ of work on the surroundings. What is the change in internal energy of the system?
-636 kJ
The volume of a sphere is given as 9.812 cm3 and the known density is 6.57 g/cm3. What is the mass of the sphere?
64.5g
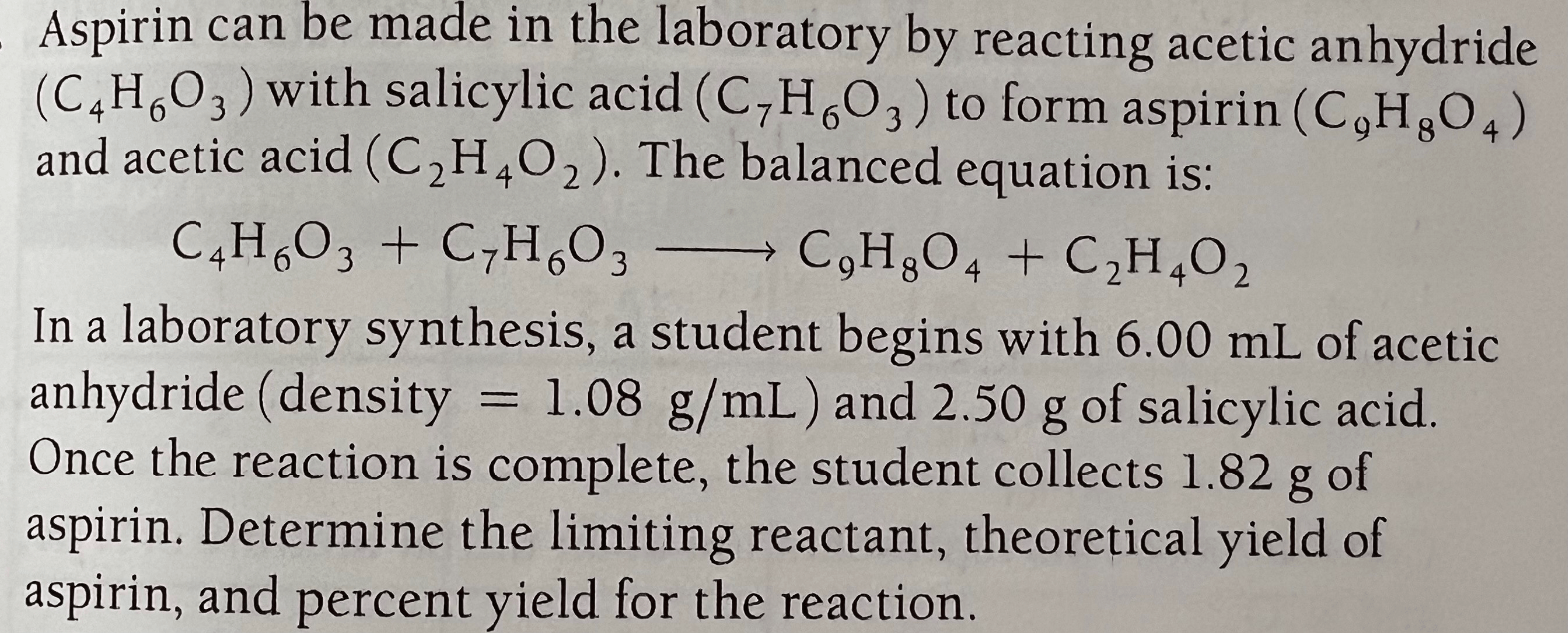
limiting reactant: C7H6O3
theoretical yield: 3.26g C9H8O4
% yield: 55.8%
What volume (in mL) of 0.0105-M HBr solution is required to titrate 125 mL of a 0.0100-M Ca(OH)2 solution?
Ca(OH)2(aq)+2HBr(aq)⟶CaBr2(aq)+2H2O(l)
238 mL HBr
H2 is produced by the reaction of 118.5 mL of a 0.8775-M solution of H3PO4 according to the following equation:
2Cr+2H3PO4⟶3H2+2CrPO4
How much H2 gas is produced (in grams)
0.3144 g H2
How many grams of gas are in 0.100 L of CO2 at 307 torr and 26 °C?
0.725 g CO2
When 1 mol of fuel burns at constant pressure it produces 6457 kJ of heat and does 27 kJ of work. What is the change in energy (E) and the change in enthalpy (H)
Change in Energy: -6484 kJ
Change in Enthalpy: -6457 kJ
Convert 76.2⁰C to ⁰F
169⁰F
Determine what element is oxidized and which is reduced are in the following reaction:
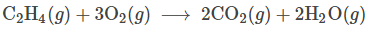
Oxidized: Carbon Reduced: Oxygen
How much will the temperature of a cup (180 g) of coffee at 95 °C be reduced when a 45 g silver spoon (specific heat 0.24 J/g °C) at 25 °C is placed in the coffee and the two are allowed to reach the same temperature? Assume that the coffee has the same density and specific heat as water (4.184 J/g*⁰C).
will drop by 1 degree
What volume (in mL) of 0.750 M hydrochloric acid solution can be prepared from the HCl produced by the reaction of 25.0 g of NaCl with excess sulfuric acid?
5.70 x 102 mL of HCl
A cylinder of O2(g) used in breathing by patients with emphysema has a volume of 3.00 L at a pressure of 10.0 atm. If the temperature of the cylinder is 28.0 °C, what mass of oxygen is in the cylinder?
38.7 g O2
How many milliliters of water at 23 °C with a density of 1.00 g/mL must be mixed with 180 mL of coffee at 95 °C so that the resulting combination will have a temperature of 60 °C? Assume that coffee and water have the same density and the same specific heat (4.184 J/g*⁰C).
170 mL
Balance the following equation:
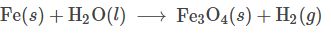
3 Fe(s) + 4 H2O (l) -> Fe3O4 (s) + 4 H2 (g)
Complete and balance the following reaction (with states of matter)
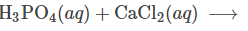
2 H3PO4(aq) + 3 CaCl2(aq) -> Ca3(PO4)2(aq) + 6H2O(l)
A 36.0–L cylinder of a gas used for calibration of blood gas analyzers in medical laboratories contains 350 g CO2, 805 g O2, and 4880 g N2. At 25 degrees C, what is the pressure in the cylinder in atmospheres?
141 atm
Toluene, C6H5CH3, is oxidized by air under carefully controlled conditions to benzoic acid, C6H5CO2H, which is used to prepare the food preservative sodium benzoate, C6H5CO2Na. What is the percent yield of a reaction that converts 1.000 kg of toluene to 1.21 kg of benzoic acid?
2C6H5CH3+3O2⟶2C6H5CO2H+2H2O
91.3%
How much faster does chlorine gas effuse than Radon gas?
1.77 times faster
A sample of 0.562 g of carbon is burned in oxygen in a bomb calorimeter, producing carbon dioxide. Assume both the reactants and products are under standard state conditions, and that the heat released is directly proportional to the enthalpy of combustion of graphite (-393.5 kJ/mol). The temperature of the calorimeter increases from 26.74 °C to 27.93 °C. What is the heat capacity of the calorimeter and its contents?
15.5 kJ/ºC
What is the final mass of Copper based on the below equation:
(6.18g + 7.321g + 781.2g - 60.0g)/((32.12g + 42.0g)*21.245g)
0.468g
What is the limiting reactant in a reaction that produces sodium chloride from 8 g of sodium and 8 g of chlorine gas?
Cl2

30.8 kg CO2
A 64.78 mL sample of an unknown nitric acid is titrated with a 0.587 M strontium hydroxide solution. The equivalence point is reached when 22.97 mL of strontium solution is added. What is the concentration of the unknown nitric acid?
0.417 M HNO3
The hydrogen gas formed in a chemical reaction is collected over water at 30.0⁰C at a total pressure of 732 mmHg. What is the partial pressure of the hydrogen gas collected in this way? If the total volume of gas collected is 722 mL, what mass of hydrogen gas is collected? Water vapor pressure at 30.0⁰C is 31.82 mmHg.
PH2=0.921 atm massH2=0.0538 g
Calculate ΔH° for the process Co3O4(s)⟶3Co(s)+2O2(g)
from the following information:
Co(s)+(1/2)O2(g)⟶CoO(s) ΔH°=−237.9kJ
3CoO(s)+(1/2)O2(g)⟶Co3O4(s) ΔH°=−177.5kJ
891.2 kJ
Write the net ionic equation for the following reaction:
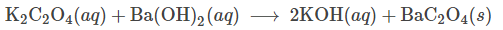
Ba2+ (aq) + C2O42- (aq) -> BaC2O4 (s)
Draw the Lewis structure with all resonance structures for sulfate. Show the formal charge, and determine electron geometry, molecular geometry, and hybridization for all central atoms, and then determine how many sigma and pi bonds are in the molecule.
See posted answer key
Determine which of the following Lewis structures is more favorable. Explain your choice. 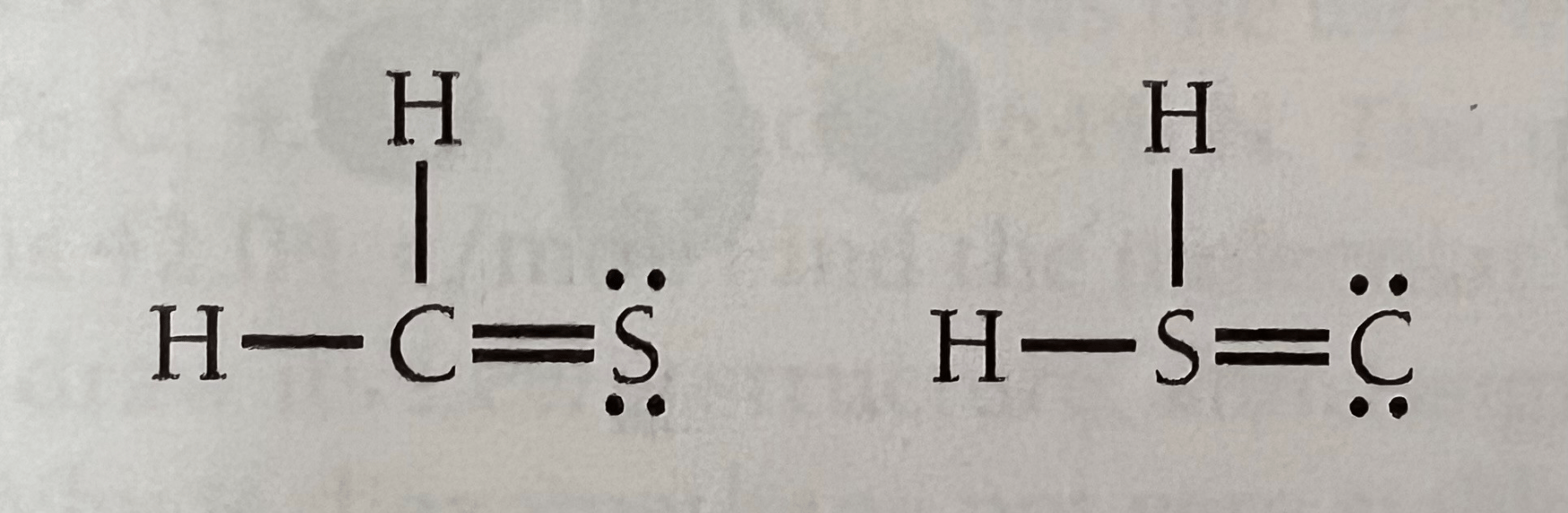
H2CS is more favorable because all formal charges are 0