Select the strongest bond:
a) N=C
b) N≡N
c) N-O
d) N-S
e) N-F
N≡N
Select the element with the highest electronegativity.
(a) Si
(b) P
(c) N
(d) O
(e) S
O
Draw the correct Lewis structure for Se
Se (6 v. electrons)
Of the bonds listed below, which is the strongest?
a) sigma bond
b) pi bond
c) single bond
d) double bond
e) triple bond
triple bond
A triple bond has ___ sigma bonds and ___ pi bonds.
1 sigma, 2 pi
Select the most polar bond:
a) C-N
b) B-Cl
c) B-F
d) C-O
e) N-O
B-F
Select the element with the lowest electronegativity:
a) Be
b) Ba
c) Al
d) Si
e) S
Ba
How many total shared electrons are around boron in BCl3?
(a) 8
(b) 7
(c) 6
(d) 3
(e) 5
3
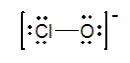
The formal charges on Cl and O in the structure shown for the ClO- ion are...
0 and -1
A neutral molecule having the general formula AX4E2 will have what type of hybridization?
(spppdd) sp3d2
Which of the following properties is least characteristic of typical ionic compounds?
a) high melting point
b) poor electrical conductor when molten
c) high boiling point
d) brittleness
e) poor electrical conductor when solid
poor electrical conductor when molten
Select the compound with the highest lattice energy
(a) BaO
(b) NaI
(c) LiBr
(d) MgO
(e) CaS
MgO
Draw the best Lewis Structure for oxygen difluoride
:)
The electronic structure of the CO32– molecule is best represented as a resonance hybrid of
___ equivalent structures.
(a) (insert number)
OR
(b) This molecule does not exhibit resonance
3
DAILY DOUBLE:
Which of the following species is planar?
(a) NH3
(b) H3O+
(c) SO32–
(d) PF3
(e) NO3–
NO3–
List 5 covalent bond properties
1) weak intermolecular forces
2) poor electrical conductor in all states
3) low melting point
4) low boiling point
5) weaker than ionic bonds
DAILY DOUBLE:
Estimate the heat of reaction at 298 K for the reaction shown, given the average bond
energies below:

Br2(g) + 3F2(g) → 2BrF3(g)
-516 kJ
Which of the following are correct lewis structures?
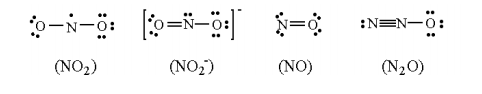
(a) NO2 and NO
(b) NO2– and NO
(c) NO and N2O
(d) NO2– and N2O
(e) All of the above are correct structures
NO2– and N2O
What is the molecular structure and electron pair geometry of KrF2 ?
linear and trigonal bipyramidal
In which of the molecules below does the central atom have an sp3d orbital hybridization?
(a) SO42–
(b) H2O
(c) CH4
(d) XeF4
(e) XeF2
XeF2
Arrange the following bonds in order of increasing bond strength.
(a) C–I < C–Br < C–Cl < C–F
(b) C–F < C–Cl < C–Br < C–I
(c) C–Br < C–I < C–Cl < C–F
(d) C–I < C–Br < C–F< C–Cl
(e) None of the above orders is correct.
C–I < C–Br < C–Cl < C–F
Arrange oxygen, sulfur, calcium, rubidium, and potassium in order of decreasing
electronegativity.
(a) O > S > Ca > Rb > K
(b) O > S > Ca > K > Rb
(c) O > S > Rb > K > Ca
(d) O > S > Rb > Ca > K
(e) None of the above orders is correct.
O > S > Ca > K > Rb
Considering all the bonds in a molecule with trigonal bipyramidal geometry, what are the bond angle(s) present?
90, 120, and 180
Which of the following BEST describes the bonding found within solid Al2O3?
(a) Strong covalent bonds between atoms with similar electronegativities
(b) Covalently bound atoms arranged in small individual molecules.
(c) Electrostatic attractions between + and - charged ions
(d) Positively charged ions covalently bound with many mobile electrons
(e) None of these
Electrostatic attractions between + and - charged ions
Which of the following molecules and ions will have a square planar geometry as predicted by the VESPR theory?
a) PCl3
b) BF4-
c) XeF4
d) BrF5
e) H3O+
XeF4