This is the base unit of temperature in the SI system.
What is kelvin?
This is the color observed in the hydrogen spectrum when the wavelength is 655 nm.
What is red?
The visible light spectrum is located between these two portions of the electromagnetic spectrum.
What are the ultraviolet and infrared?
This many valence electrons are present in the structure H2S.
What are 8? 2(1) + 6 = 8
This is the bond angle for the linear molecular geometry.
What is 180?
This is the factor for the prefix nano.
What is 10-9?
This is the color observed in the hydrogen spectrum when the light produced by an electron making the transition from n=6 to n=2 energy level in a hydrogen spectrum.
What is blue?
A sodium streetlight gives off yellow light with a wavelength of 589 nm (1 nm = 1 x 10-9 m). The light has this frequency.
What is 5.09 x 1014 s-1
This is the central atom hybridization of PF5.
What is sp3d?
Ammonium is an important structure in animal nutrition. If this is the Lewis structure, what molecular structure would be expected? 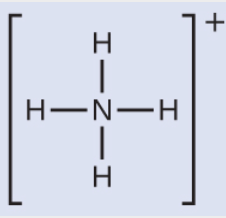
What is trigonal bipyramidal.
This is the density of lead if a cube has an edge length of 2.00 cm and a mass of 90.7 g.
What is 11.3 g/cm3?
This is the color observed AND wavelength of the light produced by an electron making the transition from n=4 to n=2.
What is green and 486 nm?
Radiation from a neon light has a wavelength of 640 nm. This is the energy of the photon being admitted.
What is 3.10 x 10-19 J.
This is the form of electron pair geometry in ClF5.
What is octahedral?
What bond angle would be expected in this structure, which has only 3 electron regions?
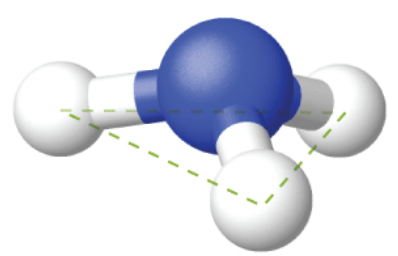
What is 120?
What is 8.9 cm3 and 7.8 g/cm3?
This is the energy in joules and the wavelength in meters of the photon produced when an electron falls from the n = 5 to the n = 3 level in a He+ ion (Z = 2 for He+).
What is 6.198 x 10-19 joules and 3.205 x 10-7 m
If a spark promotes the electron in a hydrogen atom into an orbit with n=3, this is the calculated energy of the electron.
What is -2.421 x 10-19 J?
This is the molecular geometry of XeF4.
What is square planar?
This is the bond angle for the tetrahedral molecular geometry.
What is 109.5?
What is 4.41 oz?
What is the infrared portion of the electromagnetic spectrum?
This is the wavelength of an electron that is traveling at a velocity of 1.000 x 107 m/s with a mass of 9.109 x 10-28.
What is 7.274 x 10-11m
This is the formal charge on the central atom and molecular polarity of BeCl2.
What is 0 (non-polar).
These are the two bond angles in the following structure.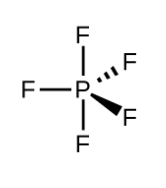
What are 120 and 90?