Rank the following from most to least acidic:
1 2 3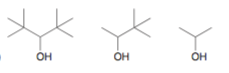
3 > 2 > 1
Which of the following compounds has a conjugated by system (if any) and explain why.
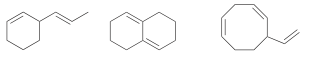
The middle compound, the pi bonds are only separated by a single bond.
What functional group is formed when a primary amine reacts with an aldehyde/ketone?
Imine 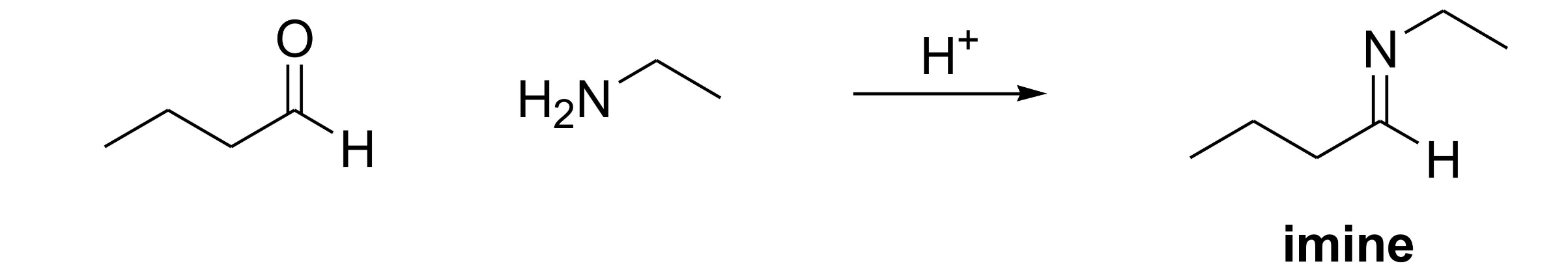
Rank the following compounds in order of increasing acidity:
1 2 3
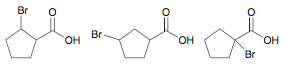
(least acidic) 2 < 1 < 3 (most acidic)
How do you list a ketone and an alcohol as a substituent on a carbon chain?
Ketone: "oxo"
Alcohol: "hydroxy"
What reagents are necessary for this transformation?
1) NaOH
2) PCC
Are halogens activating or deactivating groups? Where do they direct (ortho/para or meta)?
Deactivating, ortho/para.
What two compounds are needed to form an enamine under acidic conditions?
An aldehyde/ketone and a secondary amine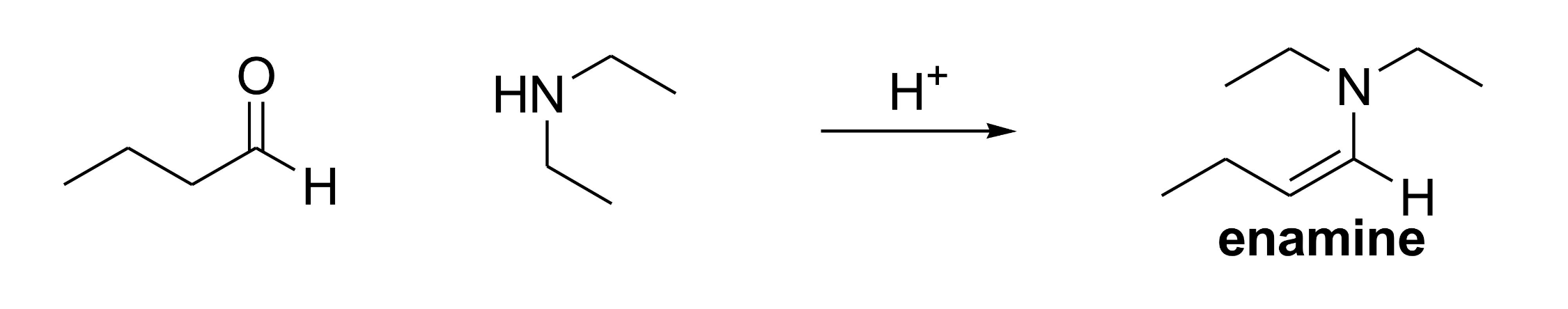
What must be present on the starting material if when reacted with Jone's (Na2Cr2O7) a carboxylic acid is formed?
Primary alcohol
Name the following compound:
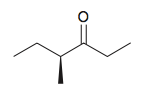
(S)-4-methylhexan-3-one
Reagent?
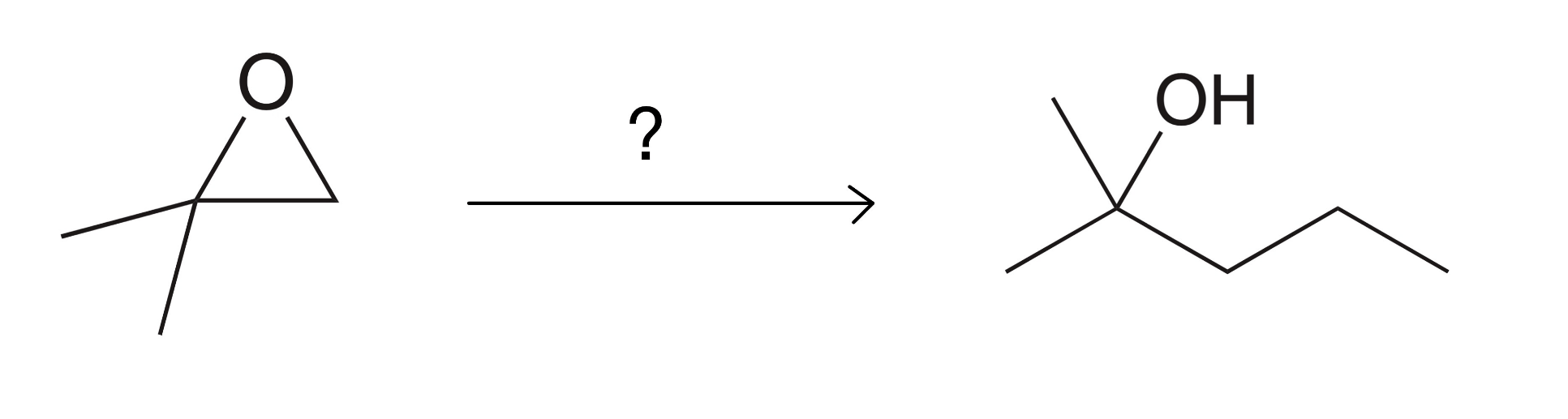
1) EtMgBr
2)H2O
Is this compound aromatic, nonaromatic or anti-aromatic? Explain.
Aromatic: cyclic/conjugated, flat, 6 pi electrons.
What reagent is needed to form an aldehyde from a terminal alkyne?
What reagent is used to convert an acid halide into a anhydride?
1. COOH
2. Pryidine
What is the priority order when listing functional groups on a compound?
aldehydes > ketones > alcohols > multiple bonds > halogens/alkyl groups
What is the major pathway (Sn1, Sn2, E1 or E2) when reacting an alcohol with NaH and a 2o RX?
E2
Which of the following compounds is the most activated towards EAS reactions? The least activated?
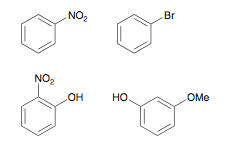
Bottom left is most activated, it contains two strong activating groups. Top right is most deactivated, it ONLY contains a deactivating group.
When using acetal formation in synthesis as a protecting group, which reagent do you need to use to convert the acetal back into the aldehyde/ketone?
H2O, H+
What is the only compound that a Gilman reagent will react with?
Name the following compound:
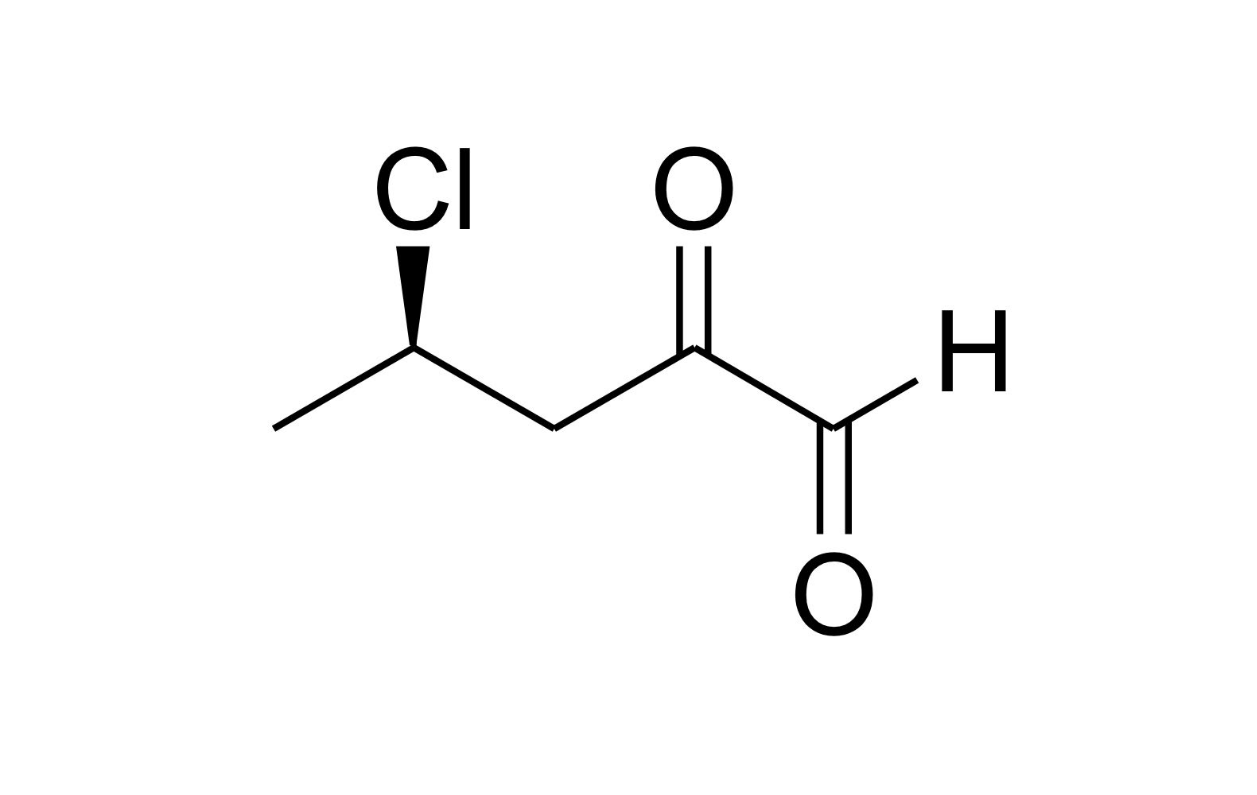
(R)-4-chloro-2-oxopentanal
What are the two reagents used to form an epoxide on an alkene?
MCPBA and Br2,H2O/NaOH
Identify the number of pi electrons in the following compound:
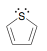
6 Pi electrons
In Wittig reactions, which isomer (E or Z) is favored when the ylide contains electron withdrawing groups?
E Isomer
How many alcohols are formed when a cyclic ester is reduced using LAH?
2 OH's
Name the following compound:
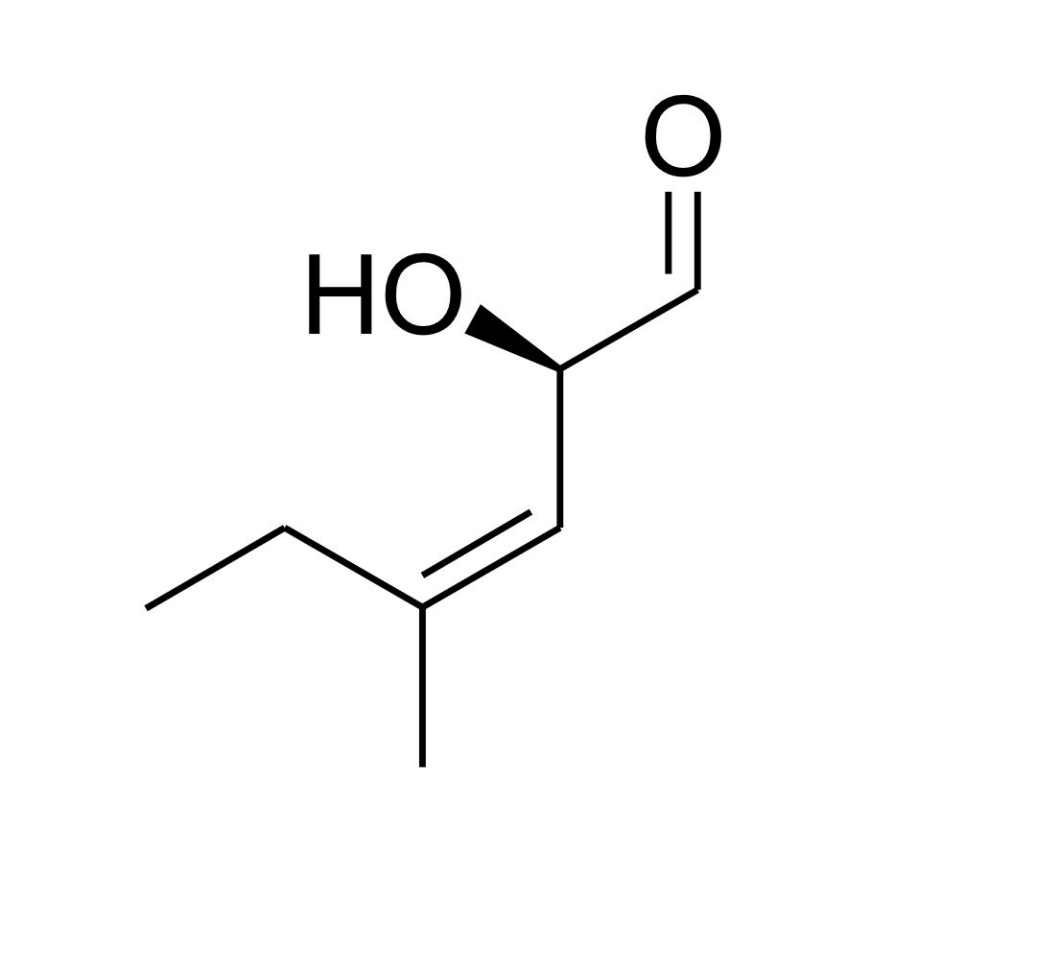
(R)-2-hydroxy-4-methylhexe-3-enal