What is the method used to measure the volume of an irregular solid?
Water Displacement
Which of the following compounds is dissolved in water?
A. H2SO4 (aq) C. H2SO4 (s)
B. O2 (g) D. C6H14 (l)
A. H2SO4
1. What is the density of an object with a mass of 20 g and a volume of 10 mL?
2 g/mL
After heating up the dark blue solid in the copper cycle lab, what color did the product turn and what was its state of matter?
Black and Solid
From which part of Chicago did drill rap originate from?
southside chicago
What is the volume of the rock?
10 mL
Which substance cannot be broken down further during a chemical reaction?
A. Ammonia (NH3) C. Argon (Ar)
B. Water (H2O) D. Hydrogen gas (H2)
C. Ar
What is the unit for density?
g/mL
What is the name of the following compound: HNO3 (aq)? Which step did we use it?
Nitric acid, step 1
How Many Hamburgers Does McDonald's Sell Every Second?
75
What is the name of this object?
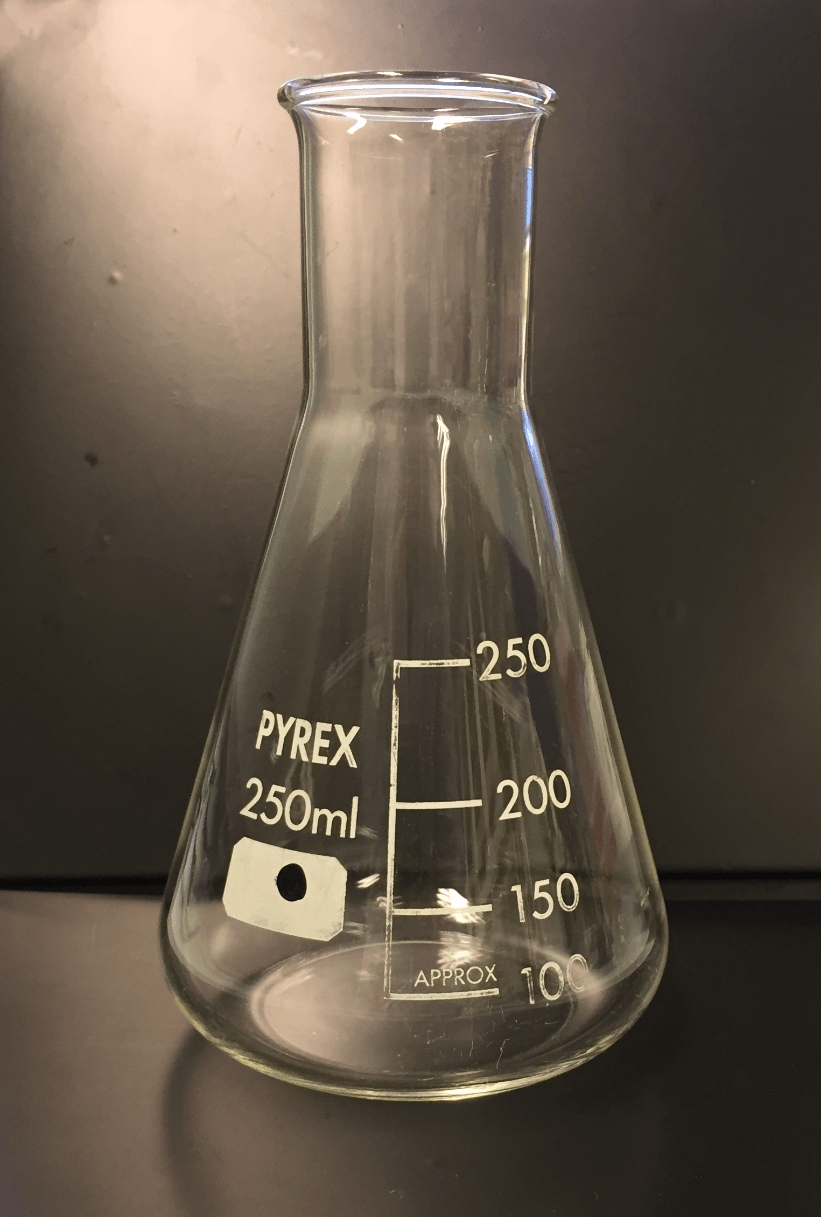
Erlenmeyer Flask
Which of the following is not considered matter?
A. Orange juice C. Air
B. Sound D. Notebook
B. Sound
What is the mass of an object with a density of 8.6 g/mL and a volume of 24 mL?
210 g
In the second step of the Copper Cycle lab, NaOH was added to a blue solution. What were the color and state of matter of the product?
Solid, Dark blue
Who Invented The Word Vomit?
William Shakespeare
What are the volumes of the liquids below?
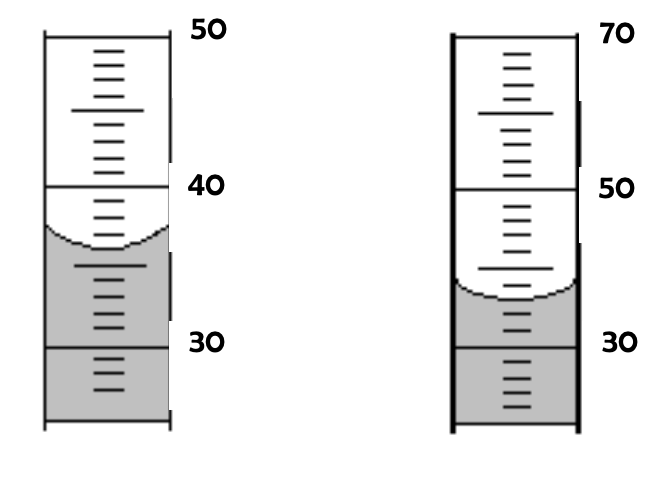
Both 36 mL
PbSO4 + AgNO3 → Ag2SO4 + ________
PbN or PbNO3
What are the two units we learned for volume?
cm3 and mL
Which two steps gave you a product like this?
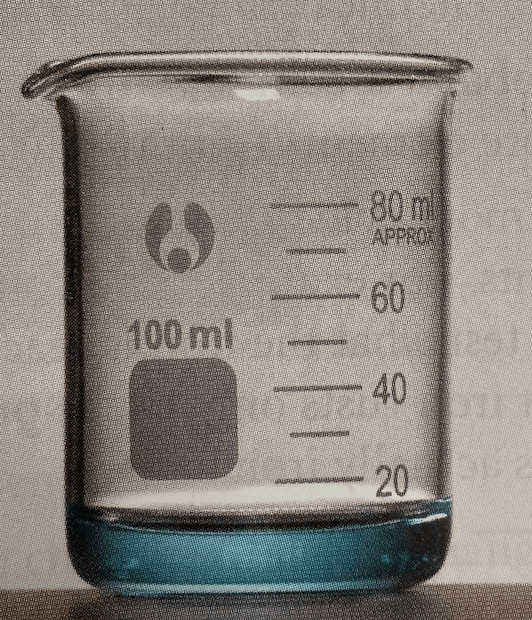
Step 1: adding nitric acid
Step 4: adding sulfuric acid
Who is the current first lady of United States of America?
Jill Biden
When measuring a mass of a solid on a watch glass, why do you need to take the mass of the watch glass alone and the mass of the watch glass with the solid?
To get the most accurate mass of your solid alone, you need to make sure your mass of solid doesn't include the watch glass.
If you started with 80 g of Na2CO3 (aq) and produced 50 g of Na2O (s), what is the mass of CO2 (g) produced?
Na2CO3 (aq) → Na2O (s) + CO2 (g)
30 g because Law of Conservation of Mass
A student has a rectangular block. It is 2 cm wide, 3 cm tall, and 20 cm long and has a mass of 600g. What is the density of the block?
5 g/cm3
Write a claim and evidence for this step of the reaction.
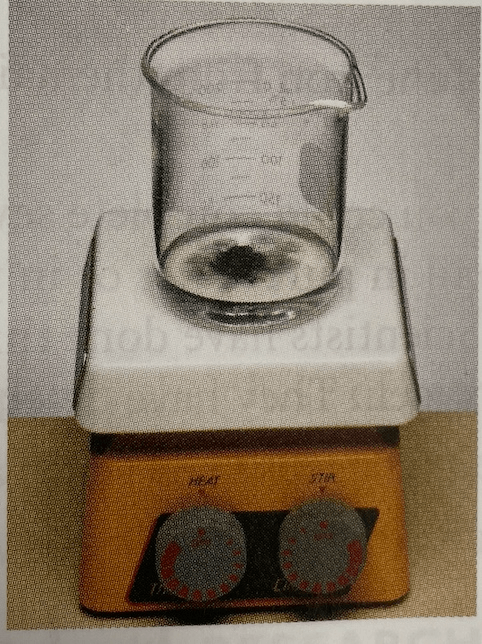
Claim: Matter was conserved in reaction 3
Evidence: The reaction started with dark blue solid and ended with a black solid.
Which rapper acted in the movie Poetic Justice?
Tupac