Equation for Molarity
What is
Molarity = (mol solute)/(Liters Solution)
Calculate the % by mass of the Substances of a solution containing 16.5g of Sucrose (C12H22O12) dissolved into 200.0g of water. Water has a density of 1.14 g/mL
What is 92.38% Water and 7.62% Sucrose
Hydrogen bonding can only occur with what 3 elements?
What is Fluorine, Oxygen, and Nitrogen
When two pure substances are mixed to form a solution, which statement always applies:
a) Heat is released
b) Heat is absorbed
c) Entropy is increased
d) Entropy is decreased
What is c) Entropy is increased
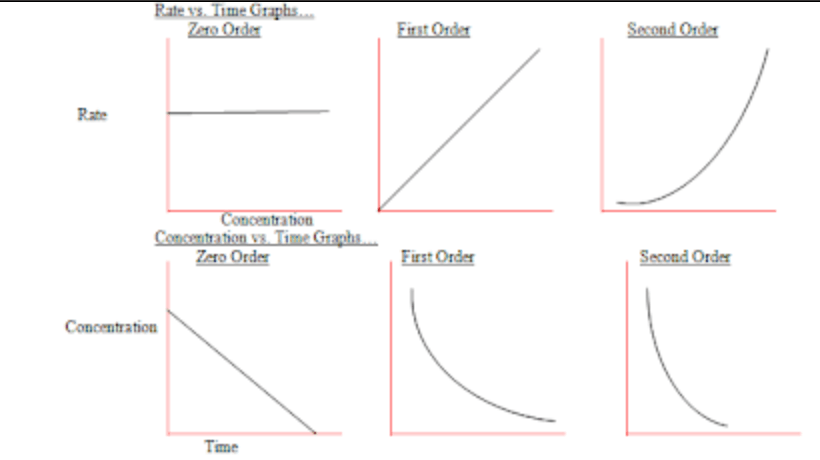
The graph for a second order reaction
Equation for mass percent?
What is
Mass Percent = (mass)/(Total Mass) *100
Calculate the Mole Fraction of the Substances of a solution containing 16.5g of Sucrose (C12H22O12) dissolved into 200.0g of water. Water has a density of 1.14 g/mL
What is 0.996 H2O and 0.00413 Sucrose
Rank the intermolecular forces from strongest to weakest
What is Ion-Dipole > H-Bonding> Dipole-Dipole > LDF
Which statement about these substances is correct?
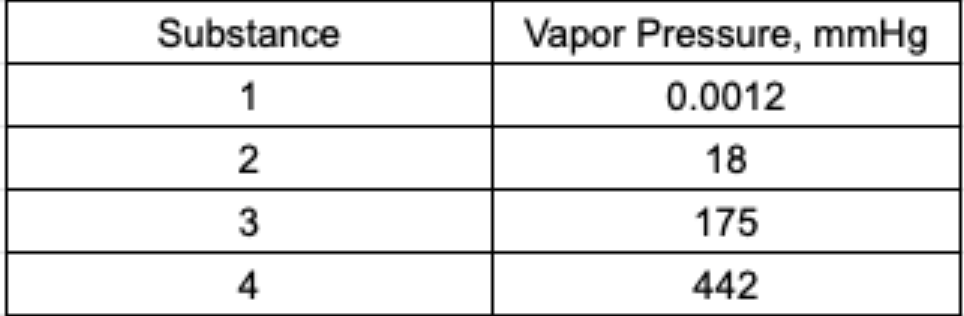
a) The boiling point of 4 should be greater than that of 2
b) The heat of vaporization of 3 should be greater than that of 2
c) The intermolecular forces between molecules of 2 should be greater than those between molecules of 1
d) The surface tension of 2 should be greater than that of 3
What is d) The surface tension of 2 should be greater than that of 3
What are the proper units for K in a second order reaction?
What is
M^ -1 s^ -1
Equation for Vapor Pressure in a Mixture
What is New VP=(Mole Fraction)VP of pure substance
What is the molality of a solution prepared by dissolving 86.9 g of diethyl ether, C4H10O, in 425 g of benzene, C6H6?
What is 2.76 m
The freezing point of an aqueous Solution of CaCl2 of 0.050m is -27°C. What is the van't Hoff Factor(i) for CaCl2 at this concentration?
What is 2.9
Which molecule has the lowest vapor pressure at room temp?
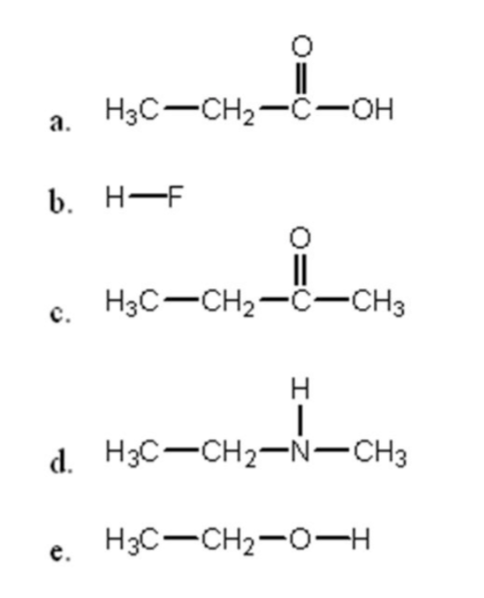
What is b) H-F
The rate law for the reaction A + B ---> C + D is first order in [A] and second order in [B]. If [A] is halved and [B] is doubled, the rate of the reaction will
What is increased by a factor of 2.
Equation for Freezing/Boiling Point elevation?
What is change in
T=Kbmi
What is the molarity of a 10.5% by mass glucose solution? The formula of glucose is C6H12O6 and the solution has a density of 1.03 g/mL ?
What is 0.600 M
26.2g of an unknown covalent compound is added to 200.0g of water. The boiling point of the water in the solution is found to be 192.6°C. Assuming that the standard boiling point of water is °C, calculate the molar mass of the unknown covalent compound. Kb=0.513°C/m
What is 25.8 g/mol
A solution contains 4.08 g of chloroform (CHCl3) and 9.29 g of acetone (CH3COCH3). The vapor pressures at 35 °C of pure chloroform and pure acetone are 295 torr and 332 torr, respectively. Assuming ideal behavior, calculate the vapor pressures of each of the components and the total vapor pressure above the solution.
what is
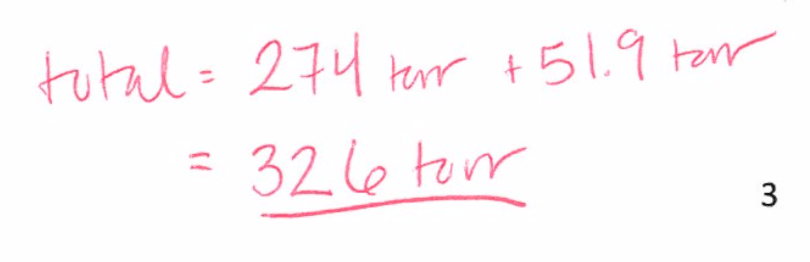
2N2O5(g) -> 4 NO2(g) + O2(g)
The rate of appearance of oxygen is found to be 0.036 M/s. What is the rate of appearance of NO2? What is the rate of disappearance of N2O5(g)?
What is Rate of appearance = 0.14 M/s NO2
and Rate of Disappearance = -0.072 M/s O2
Equation for Related Rates
what is
Rate=K[Con.A]^x[Con.B]^y
Nitric acid is usually purchased in concentrated form that is 70.3% HNO3 by mass and has a density of 1.41 g/mL. How many mL of concentrated solution would you take to prepare 1.50 L of 0.125 M HNO3 by mixing with water
What is 11.9 mL of Solution
Tert-Butyl alcohol is a solvent with a Kf of 9.10 °C/m and a freezing point of 25.5 °C. When 0.912 g of an unknown colorless liquid was dissolved in 12.1 g of tert-butyl alcohol, the solution froze at 15.3 °C. What is the molar mass of the unknown? (Assume i = 1)
What is 67.3 g/mol
Water at 37°C has a vapor pressure of 47.1 Torr. Calculate the vapor pressure of water if 50.0 grams of glucose (C6H12O6) is added to 100.0mL of water. The density of water at 37°C is 0.993 g/mL.
What is 44.8 Torr
NO2 (g) + CO (g) --> NO (g) + CO2 (g)
What is the complete rate law of the components mentioned below?
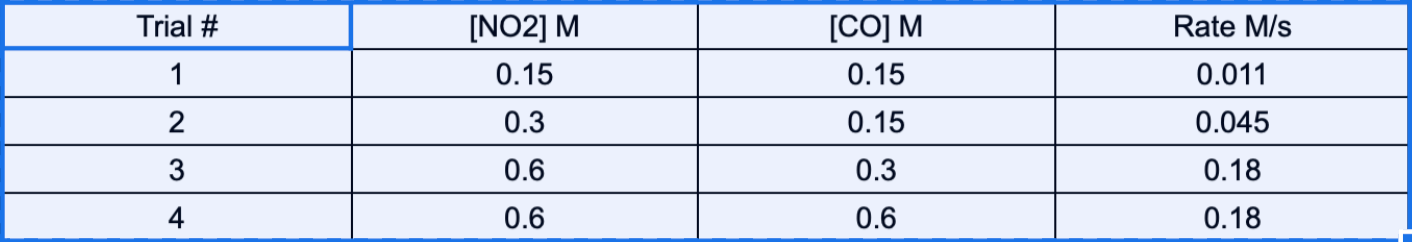
What is
Rate=0.031[NO2]^1[O2]^2