Chemistry is the study of ________________
Matter
Give the correct symbol for an alpha particle
42He
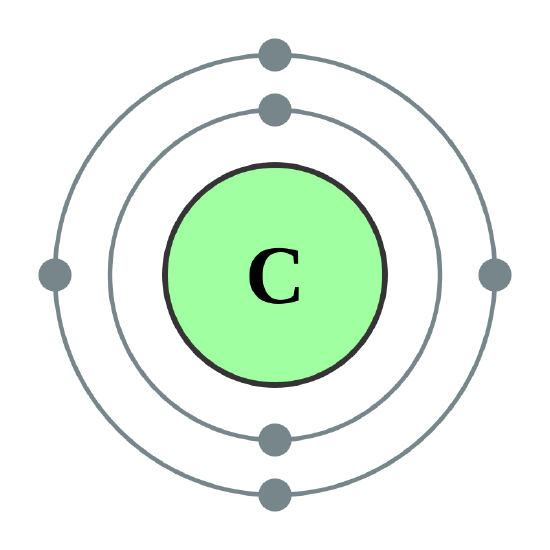
Draw the Bohr model of carbon
The majority of the elements on the periodic table are _______________ (metals/nonmetals)
Metals
Which of the following are positive ions: Cations or Anions
Cations
Give an example of a physical change and a chemical change
Answers will vary (Examples below)
Physical change: boiling water, ripping up paper, etc.
Chemical change: Rotting, metal rusting, etc.
0-1e
How many protons, electrons, and neutrons does sodium have?
11 protons, 11 electrons, 12 neutrons
Give the electron configuration of Ca
1s22s22p63s23p64s2
Which of the following are negative ions: Cations or Anions
Anions
Compounds: More than one element (Ex: MgO)
Element: Only one thing (Mg)
Answers will vary
Give the correct symbol for a gamma wave
00y
What is an isotope?
Atoms of the same element that have different number of neutrons and different mass numbers
What is electronegativity? What is the most electronegative element?
The ability to attract an electron; F
Which type of atom will tend to LOSE electrons, cation or anion?
Cation (+, LOSING negative charge, losing electrons)
Give the definition of density
How compact the mass is per unit of volume of an object
Given a nuclear reaction below, what particle would be needed to complete this nuclear reaction?
23892 U---> 23490Th + __________
42He
What does the number 35 in chlorine-35 represent?
Mass number
How does atomic size (or radius) change from left to right on the periodic talbe?
Decreases from left to right
Give the difference between an ionic and covalent compound
Ionic - metal bonded to nonmetal
Covalent - 2 nonmetals bonded together
What is the density of an object having a mass of 4.0 grams, and a volume of 12.5 cm3?
d = 0.32 g/cm3
Which type of nuclear emission is the most dangerous; alpha, beta, or gamma?
Given the data table, calculate the average atomic mass of lithium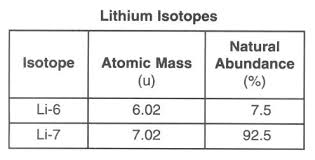
6.94
Why don't the noble gasses react with other elements?
They have a full octet; they have 8 valence electrons and are stable, they do not need any more electrons from other elements
Give a property of an ionic compound that was demonstrated in lab
Answers will vary. Examples:
High melting point, crystal structure, conducts electricity, etc.