Type of chemical reaction? Mg(OH)2 → Mg + 2OH
Decomposition
What does the variable 'm' represent in the heat transfer equation? Which units are used?
Mass and grams (g)
Name at least 2 greenhouse gases
water vapor, carbon dioxide (CO2), methane (CH4), nitrous oxide (N2O), etc.
Define an endothermic reaction
A reaction that absorbs heat energy
Is the calculation for bond enthalpy
a. enthalpy = bond enthalpy reactants - bond enthalpy products
b. enthalpy = bond enthalpy products - bond enthalpy reactants
a. enthalpy = bond enthalpy reactants - bond enthalpy products
Enthalpy = BER - BEP
Enthalpy = Make - Break
What are the 6 'types of chemical reactions'
Synthesis, decomposition, single replacement, double replacement, combustion, neutralization
What is the specific heat capacity (c) of water? (+10 pts for units)
4.18 J/gºC
Predict the products of this reaction
CH4 + O2 →
CH4 + O2 → H2O + CO2
Are combustion reactions endothermic or exothermic?
Exothermic
Draw this organic compound: C4H8

Write the type of reaction and predict the products of this reaction : NaCl + Mg →
Single replacement
NaCl + Mg → MgCl2 + Na
If a 500 g piece of copper (c=0.39J/g°C) experiences a temperature change of 30°C, what is the heat transfer (Q)?
5850 J
Name the 3 major types of fossil fuels
Coal, oil and natural gas
Is water evaporating endothermic or exothermic?
Endothermic: energy is being absorbed by the water
Calculate the bond enthalpy of this reaction
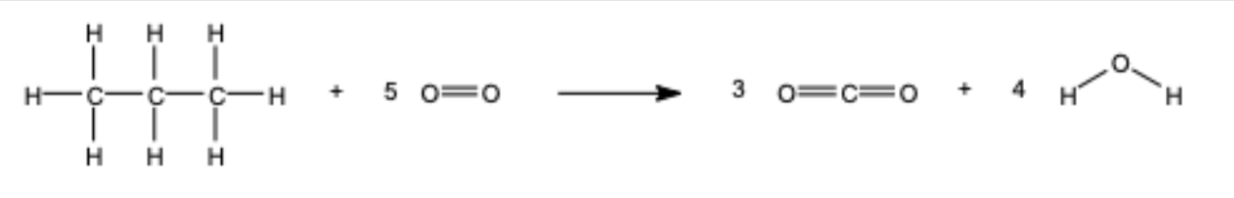
ΔH = Make - Break
= 6 494 - 8 528 = -2 034 kJ/mol
Balance this chemical equation
Zn + HCl → ZnCl2 + H2
Zn + 2HCl → ZnCl2 + H2
What is the device that is used in chemistry to insulate a chemical reaction called?
How many carbon to hydrogen bonds are there in Butane (C4H10)?
10 C-H bonds
Draw the potential energy diagram (graph) for an exothermic reaction, label Enthalpy (ΔH) and Activation energy (Ea)

Calculate the bond enthalpy for the reaction.
Is the reaction endothermic or exothermic?
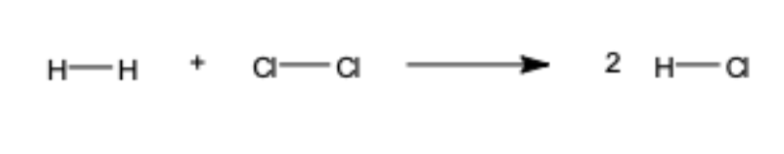
ΔH = Make - Break
= 678 - 862 = -184 kJ/mol
Exothermic
Balance this chemical equation
Fe + H2O → Fe3O4 + H2
3Fe + 4H2O → Fe3O4 + 4H2
If 300 J of heat is added to a 250 g piece of aluminum with a temperature change of 20°C, what is the specific heat (c) of aluminum?
0.06 J/gºC
Name the chemical compound found in the majority of automobile fuel (not gasoline)
Octane
Is photosynthesis endothermic or exothermic? Write out the chemical equation for photosynthesis and include '+Energy' on the correct side of the equation.
Endothermic:
H2O + CO2 + Energy → C6H12O6 + O2
Calculate the enthalpy of combustion of ethane (C₂H₆) using average bond enthalpies
1. write combustion equation
C₂H₆ + O₂ → CO₂ + H₂O
2. Balance
2C₂H₆ + 7O₂ → 4CO₂ + 6H₂O
3. Draw
4. Calculate:
4 573 - 5 994 = -1 421 kJ/mol