what is an ionic bond?
it happens when one or more electrons from one atom are transferred to another atom
what is a covalent bond?
when an electron is shared between two bonded atoms.
what is the name of PbO?
what is the formula for Hexaboron Monosilicide?
B6Si
what is Lewis dot symbol?
They are drawn to show only the valence electrons in an atom.
Name 3 physical properties?
Crystalline solids
soluble in water
high melting points
high conductivity
what are the types of covalent bonds?
single, double, triple bond
what is the name for MgBr2?
magnesium bromide.
what is the formula for Zinc(II)hydroxide?
Zn(OH)2
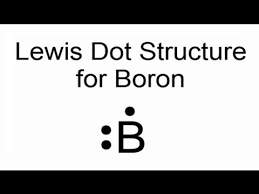
Lewis dot symbol for Boron?
what type of elements does ionic compound contain?
metal and non-metals.
Name 3 physical properties
low melting point
low conductivity in water
some insoluble.
what is the name for Li3(PO4)?
Lithium phosphate.
what is the formula for calcium and chlorine? Ionic or covalent?
CaCl2 .
Ionic compound
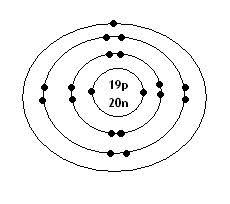
what is the Bohr model for Potassium?
what is an ionic compound?
Are composed of large numbers of positive ions and negative ions so that the amount of positive charge equals the negative charge.
what type of element does covalent compound contain?
Non-metals only
what is the name of N2O5?
Dinitrogen Pentoxide.
what is the formula of potassium carbonate?
K2CO3
what is the valence electrons behavior of a covalent compound?
the valence electrons are shared in order to achieve atoms.
ionic compound is composed of what particles?
positive and negative ions.
what is the name for Be(CH3OO)2?
Beryllium Acetate.
what is the formula for chromium (II) Fluoride?
CrF2
What is the valence electron behavior of an ionic compound?
the valence electrons are transferred from metals to non-metals.