![]() How many protons does Carbon-14 have?
How many protons does Carbon-14 have?
6 protons
The relative atomic mass of is Neon 20.179 amu.
The known Neon isotopes are:
N-20, N-21, and N-22.
Which Neon isotope is most abundant?
Ne-20
What is the density of a liquid that weighs 22g and has a volume of 14mL?
1.57 g/mol
 What is the atomic mass of this Neon isotope?
What is the atomic mass of this Neon isotope?
21 amu
Nitrogen is made up of two isotopes,
N-14 (14.003 amu)
N-15 (15.001 amu)
The percent abundance of each isotope:
N-14 (99.63%)
N-15 (0.07%)
14.007 amu
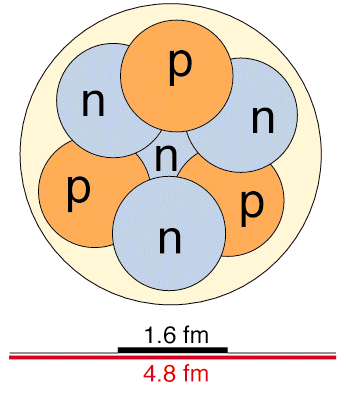 What element is this based on the picture?
What element is this based on the picture?
Lithium (3 protons)
True or false?
If I change the neutrons of an atom, then the element name changes.
FALSE
If you change the PROTONS, then the element changes
A sample of naturally occurring silicon consists Si-28 (amu = 27.9769), Si-29 (amu = 28.9765) and Si-30 (amu = 29.9738).
The natural abundance of Silicon:
Si-29 is 4.67%
Si-28 is 3.10%
Si-30 is 92.23%
Find the relative atomic mass of silicon
28.085 amu
How many protons, neutrons and electrons would Argon have?
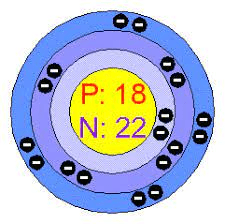
18 electrons too
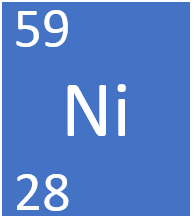 How many Protons and Neutrons does Nickel-59 (Ni-59) have?
How many Protons and Neutrons does Nickel-59 (Ni-59) have?
28 protons
59-28=
31 neutrons
Potassium has 2 common isotopes:
K-39 (6.73% abundance)
K-41 (93.27% abundance)
Average atomic mass of K: 39.098 amu
If K-39 has a mass of 38.96 amu, what is the mass of K-41?
40.96 amu
A student measure the temperature of boiling water to be 97.5 C at STP (Normal Conditions)
What would his % error be if water normally boils at 100 C?
2% error
What are the protons, neutrons, atomic mass, eletrons and Element for : Fe-56
Protons: 26
Neutrons: 30
Electrons: 26
Atomic Mass: 56
Element: Iron
Chlorine is made up of two isotopes, Cl-35 (34.969 amu) and Cl-37 (36.966 amu).
Given chlorine's atomic weight of 35.453 amu, what is the percent abundance of each isotope?
Cl-35: 75.76%
Cl-37: 24.24%
The element Sodium has a known relative mass of 22.98 amu. You did an experiment and found a mass of 21.74 amu. What was your percent error?
5.40% error