How many chemical changes are listed below?
a. rusting of a metal can
b. boiling a cup of water
c. crushing an aspirin
d. digesting a candy bar
e. exploding of nitroglycerin
3 - a, d, e
Clyde Clumsy was directed to weigh a 500.0 g mass on the balance. After diligently goofing off for ten minutes, he quickly weighed the object and reported 458.0 g. Find the percent error involved.
The average atomic mass of a sample of an element B is 10.8. What are the percentages of B-10 and B-11?
B-10 = 20% and B-11 = 80%
In which country are the Dolomites?
:max_bytes(150000):strip_icc()/header-DOLOMITESTG0422-fbe3eee35f6347f895af184866d0523d.jpg)
Italy
We wanted to know who killed our dear friend, Atom Davictim. We found out that his wife, Molly Davictim, killed him with Strychine, which she picked up on her trip to India. What analytical chemistry technique were we understanding by completing this activity?
mass spectrometry
Classify each as a pure substance or a mixture. If a mixture, indicate whether it is homogeneous or heterogeneous.
a. rice pudding 
b. magnesium
a. heterogeneous mixture
b. pure substance
What type of quantity (for example, length, volume, density) do the following units indicate?
a. cm2
b. mg/L
c. nm
d. K
a. area
b. density
c. length
d. temperature
Select all that apply. Which pair(s) of symbols represent(s) nuclei that have the same number of neutrons?
a. 5626Fe and 5828Ni
b. 5826Fe and 5626Fe2+
c. 5727Co and 5728Ni
d. 5728Ni and 5828Ni
A.
Both have 30 neutrons
In which country would you find the Batu Caves?

Malaysia
In class, we used a red laser pointer and a green laser pointer and observed what happened when shined on a pink object. We observed a color change when the green light shined on the pink, but no matter how bright the red light, we did not observe a color change. What was this demonstrating?
light's particle nature
You are in the chemistry lab preparing for a lab. This experiment requires 45.0 grams of a substance called ethylene glycol, a liquid whose density is 1.114 g/mL. You realize that all the electronic scales in the lab are broken. You have no way to obtain a scale of any sort. You do, however, have the other equipment that has been used in Ms. Long’s chemistry class so far this year. State what equipment you would use and discuss how you would use the equipment to obtain 45.0 grams of ethylene glycol.
40.4 mL of ethylene glycol using a graduated cylinder
How many liters of wine can be held in a wine barrel whose capacity is 124 quarts?

1.17 x 102 L or 117 L
Considering the visible portion of the electromagnetic spectrum only, which statement is true? Select all true statements.
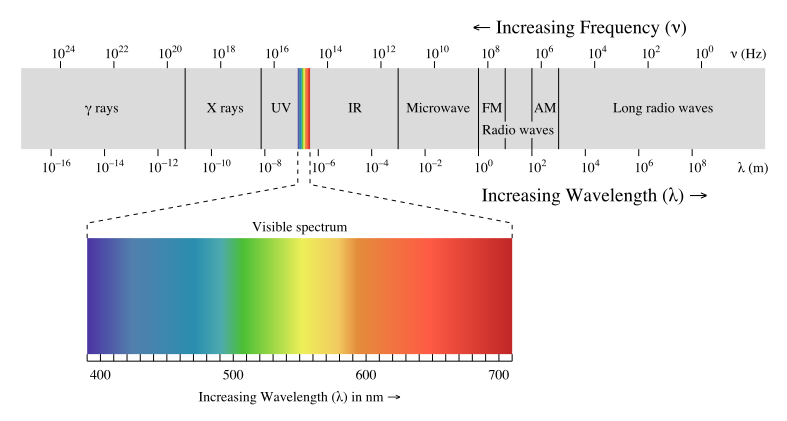
a. The wavelength of green is larger than that of purple light
b. The frequency of red is smaller than that of blue light
c. The energy of purple is greater than that of orange light
d. The speed of purple is greater than that of green light
A, B, and C
In which country is Banff National Park, home to Rocky Mountain peaks, turquoise glacial lakes, wildlife, and a picture-perfect mountain town?

Canada
In class, you had an open system with vinegar and baking soda and a closed system with vinegar and baking soda. Both systems were placed on the scale to measure initial mass and final mass. What was being observed here?

law of conservation of mass or open system vs closed system
Which diagram(s) show(s) the correct way to measure with a graduated cylinder? Select all that apply.

A.
As an instructor is preparing for an experiment, he requires 225 g phosphoric acid. The only container readily available is a 150-mL Erlenmeyer flask. Is it large enough to contain the acid, the density of which is 1.83 g/mL? Show your work.
Yes, because the acid's volume will be 123 mL
Which of the following is a false statement about the photoelectric effect?
a. Electrons will not be ejected from a metal unless light of a certain energy or higher shines on it
b. When enough photons are absorbed by the metal, an electron will be ejected from the surface
c. A single photon can eject an electron if it has enough energy
d. A dim light can eject an electron from a metal as long as the light’s frequency is high enough
b. When enough photons are absorbed by the metal, an electron will be ejected from the surface
In which country would you find Uluru, a large sandstone sacred rock?

Australia
How many milliliters of coffee can be obtained from 1 g of coffee beans? Report your answer using one significant figure.
Note: A pound (lb) of coffee beans yields 50 cups of coffee (4 cups = 1 qt).
30 mL
Name all four pieces of equipment.


tripod, wire gauze, alcohol burner, evaporation dish
The recommended adult dose of Elixophyllin, a drug used to treat asthma, is 6 mg/kg of body mass. Calculate the dose in milligrams for a 185 pound person. Report your answer using 1 significant figure.

5 x 102 mg
It takes 7.21 x 10-19 J of energy to remove an electron from an iron atom. Would light with a wavelength of 3.21 x 10-2 m be able to remove an electron from an iron atom? Explain.
No, the maximum wavelength of light that can remove an electron is 2.76 x 10-7 m and this wavelength is longer, meaning the frequency and energy will not be great enough
In which country would you find the Appalachian Mountains?

United States
A doctor is prescribing Drug X to a 72.7 kg patient. The doctor orders 5 milligrams per pound of patient weight of Drug X to be given per dosage. The supply of the drug is a solution, meaning the drug has been dissolved in water (not in pill or tablet form). The density of the solution is 0.9 g/mL. In other words, 1 mL of the solution contains 0.9 g of Drug X.
How many mL of solution need to be given to the patient per dose? Report your answer using 3 significant figures.
