How many sig figs does this number have? 0.00250
What is 3?
Write in scientific notation.
- 802,347,894 s
What is 8.02347894 x10^2?
Si unit for mass
What is kilogram?
5578m to km
What is 5.578 km?
units for measuring the density of a solid
What is g/cm^3 ?
How many sig figs?
- 0.500
What is 3?
Write in scientific notation.
0.000421
What is 4.21 x 10^-4g ?
Cubic centimeters is a unit for density? True or false
What is false?
.032kg to g
What is 32g
A block of aluminum has the following dimensions:
Length = 0.50 cm Width = 2.50 cm Height = 2.00 cm Mass = 6.75 g
- What is the density of aluminum? (sigfigs)
2.7 g/cm^3
Calculate
- 3.46 m/1.82 s
What is 1.90 m/s ?
Write in standard notation
1.61 x 10^3
What is 1610?
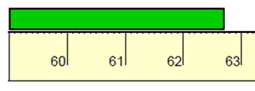 How many Centimeters?
How many Centimeters?
(include units)
62.7cm
140gal to kl
What is 0.53kl?
How do measure the volume of a cube?
what is L x W x h ?
Calculate
- 2.18 + 5.621 + 1.5870 – 1.8
What is 7.6?
Write in standard notation
3.145 x 10^-6
What is 0.000003145?
The SI unit for temperature
What is Kelvin?
182 lbs to kg
82.77kg
Mass- 147g
density- 7.00 g/ml
What is the volume?
41.0 ml
Calculate
- (238.5 + 456.18) × 0.810
What is 563?
Why do we use scientific notation?
To express very large or very small numbers.
Arrange in order from smallest to largest.
Centi, mega, nano, kilo,milli, deka, deci, micro
What is nano, micro, milli, centi, deci, deka, kilo, mega?
55 km/hr to m/s
What is 15 m/s?
What does the slope of a mass vs volume graph tell use about our substance?
What is its density?