What is the equation for density?
D= m/v
What is the rule for adding or subtracting numbers in scientific notation?
Must make the exponents the same
How many significant figures are in 4570
3
How many sig fig are in 3.00 x 10-3
3
Convert 3.4 kilograms to grams.
3,400 grams
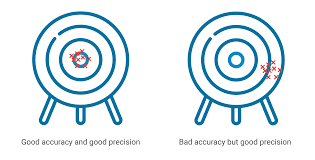
Draw accuracy vs precision on the whiteboard
What are the rules for multiplying and dividing in scientific notation?
M: Multiply coefficient, add exponents
D: Divide coefficient, and subtract exponents
How many significant figures are in 43,000.00
7
Round 4.7654 x 105 to 3 sig fig
4.77 x 105
how many hours are in a year?
8,760 hours
Calculate percent error
Experimental: 24.3 mL
Accepted 26.2 mL
7.25%
(5.0 x 105) + (2.0 x 104)
5.2 x 105
Round 64,343,450,345 to 4 sig fig
64,340,000,000
(5.3 x 103) + (3.5 x 102)
5.7 x 103
Convert 650 Liters to deciliters.
6,500 dL
Convert 32 degrees celsius to degrees kelvin.
305 degrees kelvin
(2.0 x 10-5)+(67.3 x 10-7)
2.673 x 10-5
Report answer with correct number of sig fig
4.5 +6.345=
10.8
(4.2 x 10-5) - (20.0 x 10-7)
4.0 x 10-5
Convert 145 lbs to grams
2.2 lbs=1 kg
65,909.1 grams
Calculate the density:
mass: 5400 mg (must convert to grams)
volume: 2 cm3
5400mg is 5.4 grams
5.4/2 = 2.7 g/cm3
(5.0 x 105) x (4.0 x 103)
____________________
2.0 x 102
20.0 x 108
__________ = 10.0 x 106
2.0 x 102
12.5 x 2.000=
25.0
(5.00 x 104) x (2 x 103)
10 x 107
Convert 2 miles to cm
1mi =5280 ft
1 in = 2.54 cm
321,868.8 cm
or
3.2 x 105